What's the best eQMS software in 2025?
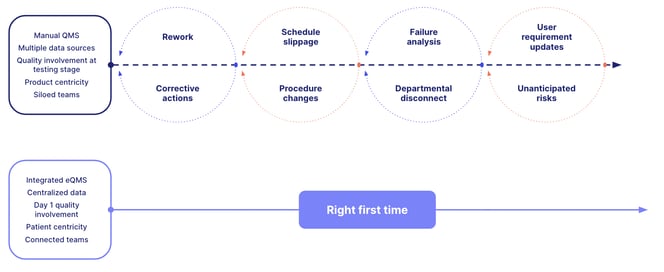
eQMS software doesn’t just make life easier for quality teams. As the life science world gets increasingly competitive, regulated and scrutinized, the right eQMS software can make the difference between success and failure.
Quality management software is an investment in your life science company's innovation, supporting the design of safe and compliant products that exceed customer expectations. So how do you determine the right quality management solutions for you?
Selecting the best quality management software requires careful consideration - not only the features, but also the user experience, training and the quality software's ability to grow with your business.
To help your search, we've compiled a list of the top quality management software systems available to life science companies in 2025.
And if you're in a hurry, watch our 4-minute eQMS software head-to-head video instead!
Table of Contents
What is eQMS software?
eQMS software is a digital tool for managing the quality and compliance requirements of the modern business.
A quality management system, or QMS, is the bundle of activities and processes that interact to guarantee the quality of your products and services.
Quality management system software, or QMS software, turns that QMS into an 'e'QMS by digitizing those activities and processes.
An eQMS is an electronic quality management system that enables quality teams to centrally manage and monitor their quality and compliance processes. eQMS software helps regulated companies, like life science, manufacturing and food organizations, create, maintain and report on key quality data elements throughout their product lifecycles.
Features like document management, training management and non-conformance tracking are just a few of the capabilities required in modern quality management systems. Software for quality management system compliance is becoming an increasingly popular way for highly regulated organizations to manage these critical quality activities.
Understanding the electronic quality management system
Quality management software, in short, is the method for structuring and running an electronic quality management system.
The primary benefit of an effective eQMS, powered by quality management system software, is that it assists you in automating the traditionally manual business processes connected to quality, compliance and product development.

In particular, businesses embrace quality management software and turn to eQMS systems to:
- Move away from paper and spreadsheets
- Connect employees to a single source of quality truth
- Save money with efficient digital quality practices
eQMS software cost
How much does eQMS software cost?
Quality management solutions for large, multinational enterprise organizations can stretch into six- and even seven-figure costs, while leaner, more focused quality management software systems for start-ups and scale-ups start in the low tens of thousands of dollars for an annual subscription.
All this is to say: electronic quality management system costs can vary significantly depending on the type of quality software you require.
As such, it's important to pinpoint your exact quality management system software requirements as you scope potential platforms.
It's also important to remember that software for quality management system compliance typically pays for itself in a matter of months - so don't be put off by the up-front investment costs.
Process efficiencies, faster pathways to market, slimmed-down audit costs and slicing the risk of regulatory fines are all very real ways for your eQMS investment to pay for itself many times over.
eQMS software requirements
Most eQMS software is designed with a particular business type, size or industry in mind. Some offer specific value for life science companies, while others are favored by food and manufacturing businesses.
A global pharmaceutical brand with an extensive product portfolio will have different eQMS software needs than a medical device start-up focused on getting to market.
Choosing quality management solutions designed specifically for organizations like your own will translate to faster implementation, stronger adoption and swifter time to value. For highly regulated companies in sectors like life science, choosing eQMS systems comes with a lot of functionality considerations.
Let's look at some examples below.
eQMS software examples
Example eQMS features include:
- Document management
- Design controls (for medical device companies)
- Risk management
- Training management
- Audit management
- Corrective and preventive action (CAPA) management
- Supplier management
On top of this core quality management solution functionality, you should consider niche features and services that could be critical to your business, such as:
- Compliance with industry document integrity standards, like ALCOA+ or the FDA's 21 CFR Part 11 e-signature requirements
- Customer support that fits your needs
- Cloud-based access for a remote, post-COVID world
- Validation: how quick and easy is it with each vendor?
Your quality compliance software should be targeted appropriately to your organization’s growth stage, budget, structure and industry. Choosing an eQMS that doesn't streamline efficiently or fit your use case can lead to wasted cost, non-compliance, or costly software bloat from overly extensive configurations.
We don't want that to happen to you, so we examined all of the top eQMS vendors on the market - from Q-Pulse to Greenlight Guru - to assemble a complete overview.
Dive into our top 5 eQMS software comparison below to find the quality management systems software that's best for you.
Best eQMS software options
1. Qualio

Our eQMS is designed to help small- to medium-sized life science companies with 11+ heads scale and bring their products to market.
Qualio is an ideal quality management software eQMS platform for start-ups facing the world's tightest regulatory requirements, from FDA and ISO demands to GxP, and is built to do 3 things especially well:
-
Be the easiest eQMS on the market to set up and use
-
Offer best-in-class content and data management
-
Connect your critical quality processes with third-party integrations and by enabling a culture of quality at your business
Qualio's eQMS features include:
And more!
Best for: Small- to mid-sized life science companies wanting eQMS software that sets up quickly, gets adopted internally, and scales with them.
Quality management solution scoping tip
You might be thinking: "this is Qualio's website, of course they'd put themselves #1!" This kind of caution and awareness of bias is important as you hunt for an eQMS vendor.
To remedy bias, always look at customer reviews to see if a prospective quality management system software vendor is really doing what they say they can do.
Pros:
- High customer satisfaction and high-touch support
- Pre-built quality and regulatory content for accelerated compliance
- Simple, intuitive UX and usability
- Only eQMS with complete in-app document lifecycle management, powered by an intuitive document editor
- Rapid set-up and validation timeframes
- Integration with other business tools like Jira and Salesforce
Cons:
- Designed specifically for life science companies
- No ERP integrations yet
- Not designed for extensive lengthy customization
- US and EMEA footprint, limited presence elsewhere
2. MasterControl
This eQMS is a well-known brand in the life sciences industry and among the most widely adopted quality management software systems in the vertical.
Current users include several major regulatory agencies and 1,000 enterprises in 30 countries.
MasterControl's eQMS functionality is designed to address compliance and quality management in product development, clinical trials, supplier management, regulatory risk management, manufacturing and post-market use cases.
Best for: MasterControl is positioned as a quality management solution for global enterprises with a large portfolio that spans the product development lifecycle. It may be the best quality management software for your organization if you need complex, highly configurable eQMS software and can dedicate significant resources toward implementation and customization.
Pros:
- High connectivity between modules and processes
- Robust features for collaboration and administration
- Users report satisfaction with efficient document management features
- The newly designed user interface is accessible and intuitive
Cons:
- Pricing is described as expensive and inflexible by adopters
- Reported issues with system and search speed
- Only offers exact match search functionality
- The web app is only compatible with Internet Explorer
- Challenging usability
3. Veeva
Veeva is a long-established eQMS provider with a large, complex cloud quality software platform to match.
It's therefore one of the best enterprise quality management software systems for larger companies to check out.
Over 1000 enterprise businesses use Veeva for their quality management software needs, and the company offers a vast range of eQMS tools and features from Vault QMS to Vault QualityDocs, Vault LIMS and Vault Station Manager.
Best for: Enterprise organizations that can commit significant budget towards product configuration and don't need rapid implementation of their eQMS.
Veeva skews towards a life science customer base, but it isn't a dedicated industry tool - and so cosmetics, chemical and consumer good companies also use their quality management system software.
Pros:
- Not industry-specific, could be broadly applied in several verticals
- Designed for enterprise organizations with scope, complexity and budget to match
- Dedicated LIMS, clinical trial and CRM tools available within suite
- Full 'bells and whistles' approach with layers of functionality for any niche user need
- GxP training courses also available
Cons:
- Expensive
- Complex, with long implementation and training times and potentially blunted adoption
- No in-app document collaboration/editing
- No pre-built system templates/content
- Some customer reports of difficulty using day-to-day
- No design control features for medical device customers
4. Greenlight Guru

Greenlight Guru is one of the best QMS software systems for medical device start-ups.
Why? It's one of the few QMS companies operating exclusively in that niche, and therefore offers a range of dedicated eQMS features for managing documents, training, design controls and other activities for compliance with FDA 21 CFR 820, ISO 13485 and other key medical device standards.
Best for: Medical device companies in their earliest days of operation, with 10 or fewer heads and with immature quality management.
Pros:
- Rigid guardrails offer a great structure for companies with very immature quality
- Designed specifically for medical device companies, with all functionality focused on start-ups in that vertical
- Low configurability makes it quick and easy to adopt
- Priced for start-ups and scale-ups
Cons:
- Lack of flexibility and GAMP 5 Category 3 set-up makes it difficult to adapt and scale
- Not suitable for larger organizations or those not manufacturing medical devices
- Few integrations
- No detailed analytics or native document building
Hear a Greenlight Guru customer explain why he moved to Qualio
5. TrackWise
Sparta Systems’ TrackWise product is an eQMS software platform designed for organizations subject to GMP requirements. The vendor positions the product as a comprehensive eQMS software solution designed to increase visibility and improve organizational efficiency in a competitive market.
The system offers the potential to integrate into a complex software ecosystem at enterprise organizations, including real-time integration with ERP, CRM, laboratory information management (LIMS), and manufacturing execution system (MES) applications.
Best for: Larger organizations seeking a robust eQMS software solution might find TrackWise is the best quality management software for them.
G2 user-reported data indicates the majority of TrackWise adopters are very large organizations. TrackWise’s potential for integration is a significant strength, but also a necessity for highly regulated life science businesses: the software’s features are not sufficient on their own to embed compliance with ISO 13485 and other life science standards.
Pros:
- Quality control software with a wide range of workflows and modules
- Scalable and designed for multi-site implementations
- Extremely flexible system supports extensive customization
Cons:
- Does not include archiving or retention features
- Does not include controls testing
- High training barrier, confusion, and functionality issues reported by users
- User experience is not very intuitive
Choosing the best eQMS software for you
Thanks to the growing life science industry, your eQMS options are growing rapidly for 2025.
Don't settle for quality management solutions that aren't purpose-built for your industry, that exceed your budget, or that demand extensive configuration.
When it comes to finally choosing the best eQMS for you, double and triple check the key factors of user-friendliness, scalability, industry compliance and cost. Additionally, make sure you understand what type of support your prospective quality management software systems offer, and how often they're updated.
By doing your research and making an informed decision, you can make sure that you choose the best quality management software that matches your company’s needs for the long term.
Today, the market offers eQMS system software that's flexible, cloud-based, and more cost-effective. The best eQMS for your company is the one that most closely aligns with your product and team specific goals and help you reach your next phase of growth.
Want to start your journey right now? 👇