What is a quality management system (QMS)?
Nobody ever asks if they need a sales team, or how a HR team works, when they set up a company. But the question, "what is a quality management system?" is one that businesses have to confront, often unexpectedly, as they set themselves up to scale and get to market.
When you think about how a business works, the first things that probably come to mind are the typical processes and departments: product development, sales, marketing, HR, and so on.
But each of these operational areas are driven by an overarching system that is often overlooked, ignored or misunderstood: the quality management system, or QMS.
We're going to dive into exactly what a QMS is, what it should look like, and - most critically - why your business absolutely needs one.
Table of Contents
What is a quality management system?
A quality management system is the bundle of processes and responsibilities that makes your business run how it’s supposed to, and ensures that the products and services that you provide satisfy your customers and your stakeholders.
In a nutshell, that's it. Your QMS is the formalized system that guides your business to its objectives and ensures the quality of what you do.
ISO defines quality as the:
"... degree to which a set of inherent characteristics of an object fulfils requirements."
The quality management system, then, is the mechanism which maximizes the ability of your 'objects' - products and services - to fulfil the requirements of the people that receive them from you and use them.
The stronger your quality management system is, and the more you invest in it, the stronger the rest of your business becomes.
In fact, according to Harvard Business Review:
Companies with highly developed [quality] spend, on average, $350m less annually fixing mistakes than companies with poorly developed [quality].
It's worth noting, too, that the strength of your QMS can depend on the tools and platforms you use to manage it. More and more quality-centric companies are turning to quality management system software to turn their quality systems into optimized, digital entities that live in the cloud.
What's in a QMS?
A good QMS is formed of some typical key ingredients as follows:
Documents
Documents structure and organize how information flows around your business, keeping everyone on the same page and working in the way they’re supposed to.
Key quality documents include your quality manual and policy, work instructions and standard operating procedures (SOPs). Document control is often viewed as the 'core' or 'baseline' of your quality management system, setting out how things should be done.
Training
An effective training program ensures everyone in your company can do their job properly. This typically happens by issuing documents such as company policies and procedures to employees, and testing if they've read and understood them.
Event management
Quality management processes like CAPA and non-conformance management make your products and services stronger, by identifying problems, weaknesses and waste, then fixing them.
These are often labelled 'event management' processes, since they involve responding to quality 'events' like complaints and defects with corrective action or preventive action to maximize product quality.
Supplier management
As businesses rely more and more on interactions with third parties for products and services, supplier management is becoming an increasingly important component of the modern QMS too.
After all, it's no good being a high-quality business if you're working with an ineffective supply chain that's dragging you down!
Download our essential guide to life science supplier management
For certain industries, other niche quality management ingredients besides these core components can come into play. Food companies, for example, need processes such as HACCP in their QMS, while medical device companies need to think about how they manage design controls.
Read up on the 9 core elements of a quality management system
The industry you operate in dictates exactly how your quality management will look, and what's in it.
But the commonalities of documents, training, event and supplier management will always be indispensable for any type of business.
Who needs a quality management system?
A functional QMS is important for any company that provides a product or service to customers. If you do any kind of business, some level of quality management is required.
But in highly regulated industries like life science or aerospace, where effective processes can be the difference between life and death, proving to a regulator that your QMS works is absolutely crucial for getting to market and selling your products.
Regulators like the FDA, EU or MHRA typically won't allow your regulated products to be marketed or used at all unless you can prove you have a functional QMS in place, and they'll conduct an audit to investigate if your quality management system works how it should.
That means digging into your document stack, asking you to prove your team are properly trained for their roles, seeing how you respond to quality events, investigating your supplier management processes, and more.
Why do you need a quality management system?
It makes sense that doing things in an ordered, consistent way that maximizes your customers' happiness is highly beneficial for your organization.
We've seen already that a quality management system, at its core, is all about making your business work how it's supposed to. Another way of saying this is that your QMS makes your business be the best it can be.
The benefits of this can be profound, including:
1. Lower operating costs
Your QMS is all about identifying wastage, weaknesses and redundancies, then fixing them.
That means leaner, stronger and more efficient processes that take less effort and cost to run.
Cutting a redundant process step, identifying a manufacturing improvement or boosting output by 10% can all be transformational moments for your business with five- or six-figure financial impacts.
2. Lower operational risk
Risk and quality management often intersect.
A robust QMS should take your operational risks, like competitors, regulatory change and process weaknesses, into account - then overcome them with proactive, well-planned processes.
The more highly regulated your company, the greater the risk you face.
A tiny product risk in the life science industry, for instance, can snowball into patient injury or death, triggering catastrophic recalls, fines, shutdowns and regulatory investigations.
A good QMS insulates your company from this risk, acting as a protective blanket that keeps you in business.
3. Boosted revenue
By minimizing costs and risk, your company's revenue will naturally increase as a result of your quality management activities.
But that's not the only thing to consider.
Aligning your colleagues around standardized, high-quality processes that keep your customers happy also boosts customer retention and spend, making them less likely to ditch your services and go elsewhere.
We can see in this chart that cutting customer churn from 20% to 5% makes your company grow 50% faster and unlock 40% more revenue over a 5-year period:
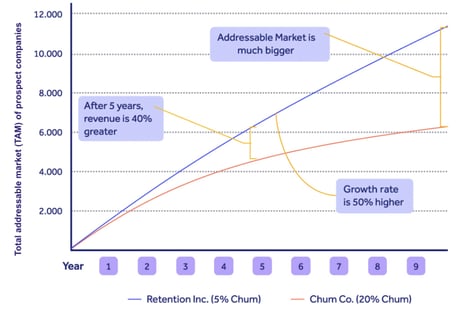
Meeting customer needs with your QMS can therefore be a vital operational weapon.
4. A stronger team
IDC's whitepaper, 'Counting the Cost of Employee Misunderstanding', found that 89% of life science companies have experienced unplanned downtime from employee misunderstanding.
85% of those have seen reputational damage as a result - and over a third have lost business.
Misunderstanding sounds like a murky and nebulous concept to tackle, but it really comes down to whether or not your employees know how to do their jobs to the best of their ability every single day.
Your quality management system is the key to fixing this problem.
Carefully documented processes, thoughtful and effective training, and a robust culture of quality all keep your teams aligned and connected - minimizing mistakes and maximizing morale.
When do you need a quality management system?
In short? As soon as humanly possible!
The QMS is the beating heart of your business and the connecting tissue ensuring all its elements work together - with customer satisfaction as the focal point.
It can be tempting to focus all your attention on your product in your company's early days, leaving 'admin' like policies and procedures for later.
This is a mistake. Committing to a quality focus from the very first days of your company, with elements like a quality policy, quality manual and clear operating procedures, will set the tone of what your organization becomes.
And companies that neglect important quality management elements like standardization and customer focus usually find that they become much harder to instil later:
"Most enterprises are organized by functions - sales, marketing, operations, maintenance, engineering, finance - that are managed independently.
Functions are typically islands of competency ruled by jealous kings, populated by antagonistic armies and separated by shark-filled seas.
These disconnects are a significant weakness."
- John S. Mitchell, "Operational excellence: journey to creating sustainable value"
Nefarious and scary things like the 'deviation spiral' can then take hold, where your teams experiment with alternative ways to do things that 'spiral' further and further away from the original intent.
Soon, it can become impossible to know what's actually happening in your company each day - and regulatory audits will become a nightmare that block your route to market.
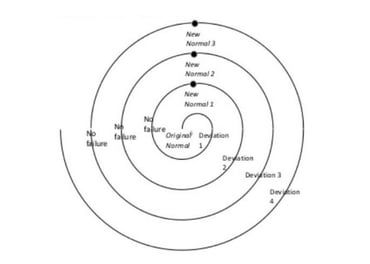
On the flip side, a proactive approach to your QMS-building will allow you to plan and feed your customer requirements into your processes early, building a quality focus quite literally into the core of your company that will stay there as you mature.
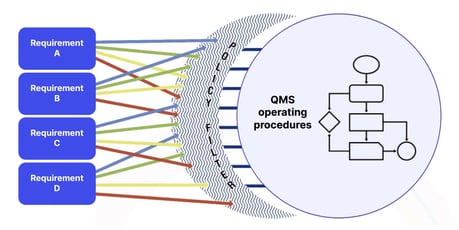
So, where should you start?
Think of the handful of core processes that define your business and what it does. Document them and give serious thought to how your QMS could make them interrelate in a standardized, consistent and customer-centric way.
Typical places to start include:
- Product design and development
- Incident management
- Risk management
- Marketing and sales
- Customer/supplier onboarding
- Competence management
Start simple and build your way up to a repeatable and measurable process approach.
Remember: a few scattered QMS ingredients to build on are better than none at all.
What does a QMS not do? 3 misconceptions
As we think about what a quality management system is, it's also worth taking some time to consider what it isn't.
Unfortunately, since the QMS remains a largely hidden and underappreciated element in a lot of businesses, there are a few misconceptions about what it does.
Misconception #1: The QMS makes rules to follow
Quality is often conflated with red tape, bureaucracy and finger-pointing compliance managers.
And while some businesses do treat quality this way, it's not how your QMS should be run.
Instead of inventing rules and procedures, then forcing everyone to follow them, your QMS should be a collaborative and business-wide connecting system that's supported by everyone.
That means your laboratory team needs to contribute to your laboratory SOPs.
Your product engineers need a big say in how your product processes are documented and run.
And you shouldn't foist an elaborate training management system onto your people team without involving them.
In short: a good QMS is a living repository for the best way to do things, agreed on and committed to by everyone in your company.
It's not the company police!
Misconception #2: The QMS is just a tool for regulatory compliance
Your quality management system is all about guaranteeing the quality and integrity of the products and services you provide.
This does serve an important regulatory and compliance role, since it allows you to prove that your drugs or medical devices are safe, effective and compliant for the markets they're entering - in turn, allowing your company to certify to quality standards like ISO 9001 or ISO 13485.
But treating your QMS purely as a 'certificate maker' and a necessary evil to make regulators like the FDA happy misses the point of what it is.
A robust, functional QMS can do so much more than give you a baseline of compliance.
Armed with the right data, your quality system opens the door to continuous quality improvement, constantly lifting your operational maturity beyond the bare minimum of compliance and strengthening your business in relation to its competitors.
So don't just think 'compliance' - think 'quality'!
As a 2019 FDA report puts it:
“Many pharmaceutical manufacturing firms have focused their efforts on compliance with cGMPs, which include standards for material systems, equipment and facilities, production, laboratory, packaging and labeling, and a quality system.
These standards, however, are foundational and set a minimum threshold that companies must achieve in order to be allowed to supply the U.S. marketplace. They do not include more advanced levels of quality management…”
If we consider the quality maturity curve, we can see how the QMS isn't just a binary tool separating compliance from non-compliance.
Rather, it's a continually evolving weapon in your company's arsenal, that can be scaled up from quality control through quality assurance to total quality management:
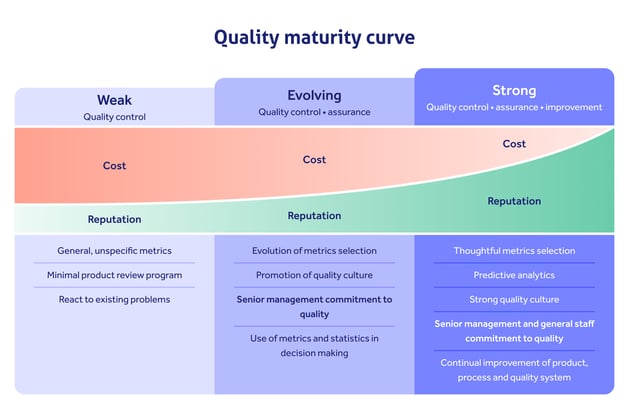
Misconception #3: The QMS is for the quality manager to run
Our 2023 life science quality professional salary report found lots of quality managers feeling siloed, cut-off and undervalued by their companies.
This is symptomatic of a broader image problem experienced by quality: that the QMS is the responsibility of the quality team, and no-one else.
As we've touched on already, the QMS should never be the remit of a single department. Since it touches the entire business, everyone in the company should be aware of their quality management obligations and commit to supporting the QMS every single day.
This can, however, be difficult in a manual and paper-based quality management system. Scattered filing cabinets and folders make it difficult for quality to become a visible part of the business culture, while quality essentials like training become frustrating, time-consuming and annoying for everyone else.
This is why more and more quality-centric organizations are turning to cloud-based electronic quality management systems (eQMS) to knit their QMS into the wider company.
Centralized information and accelerated digital processes make it much simpler for all layers of a company, from laboratory to boardroom, to access and support the QMS.
The QMS then becomes what it should be: a central cog of the organization, guided but not run by the quality team.
Conclusion: what is a QMS?
If you're asking the question, "what is a quality management system?", your company is probably in the earliest stages of its operational maturity.
That's not a bad thing. Building your QMS from scratch is a golden opportunity to turn a blank canvas into a real driver of continuous business improvement.
Careful, thoughtful planning of your quality management ingredients, and the tools you'll use to manage them, will define your business operation and set you up for long-term success. Access our quality management resources to start your research.
So, what is a quality management system? It's the key to hitting your operational goals. The best time to start building one is right now!
