Good distribution practice: understanding GDP compliance
Good distribution practice, or GDP, is a key subset of the GxP standards required in regulated industries such as life science.
GDP compliance is essential if your business is involved in the storage, transportation or handling of medicinal product within a pharmaceutical or biotech supply chain.
Good distribution practice guidelines were developed to ensure the quality, integrity and safety of medicinal product as it is physically moved from manufacturer to patient.
GDP compliance demands airtight and constant adherence to the ten 'chapters' of good distribution practice expectations.
Let's dive into how to embed GDP compliance and make good distribution practice a natural and automatic part of how your business operates.
What is good distribution practice?
It's not enough to just make safe and effective life science products.
A vaccine or tablet developed with good manufacturing practice (GMP) in a state-of-the-art facility, that's then distributed through a dirty, damaging and uncontrolled supply chain, is as unsafe for use by the time it reaches the patient as a product made without any GMP oversight at all.
It's for this reason that GDP compliance and GDP certification is a vital regulatory requirement for wholesale licence and authorization holders.
GDP in the pharmaceutical industry
GDP good distribution practice is a critical part of how the pharmaceutical industry operates, and should be baked into your pharmaceutical quality management system.
GDP guidelines were established to prevent contamination, falsification, and other risks to the quality and integrity of pharmaceutical products, from APIs to FDFs, as they move through the supply chain on their way to patients.
Good distribution practice should be seen as part of the overarching umbrella of GxP which governs the quality and integrity requirements of pharma businesses.
Although good distribution practice is expected and enforced in the US by the FDA, GDP compliance requirements are more comprehensively defined and enforced as an individual discipline in European legislation and guidance documents, so it's these that form the basis of this article.
GDP certification
GDP is so critical for pharmaceutical regulation that you'll need certification in the EU to prove you've embedded it into your operation.
Any of the EU's 'competent authorities' can issue GDP certification to a wholesale distributor within 90 days of a 'satisfactory inspection outcome'.
Your GDP certification will also be logged in the Union database for traceability and recording of your compliance with good distribution practice expectations.
GMP vs GDP
Since both good manufacturing and distribution practice fall under the umbrella of GxP compliance, there's often some confusion about the distinction between the two.
Both are crucial for modern pharmaceutical companies, so the GMP vs GDP binary isn't much of a choice.
Where GMP, or 'current' cGMP, focuses on how drugs are made, good distribution practice focuses on how they're moved and distributed.
There is some overlap in the kind of activities you'll need to perform. FDA 21 CFR Part 211, for instance, is the key cGMP standard for US pharma businesses, and the facility requirements mapped out in its Subpart C contain some of the same areas of emphasis as GDP compliance guidelines.
The focus on properly designed spaces, on good storage and maintenance, and on clear traceability of product with receipt, acceptance and identification procedures are all key elements also required for GDP compliance and GDP certification.
The main area of difference for GDP vs GMP comes with the areas of responsibility.
GMP, obviously, falls with the manufacturer. International pharmaceutical standards like ICH Q8, Q9 and Q10 are all geared towards maximizing quality and risk control in the manufacturing environment.
GDP compliance obligations rest with onward wholesale distributors and those involved in the manufacturer's supply chain.
Principles of good distribution practice
The best way to understand and unpick the key principles of good distribution practice is to examine the ten 'chapters' of GDP compliance mapped out by the EU.
These chapters form the most comprehensive and widely recognized standards of good distribution practice expectations.
Quality management system
Establishing and maintaining a robust, well-documented quality management system to ensure constant operational compliance with GDP requirements.
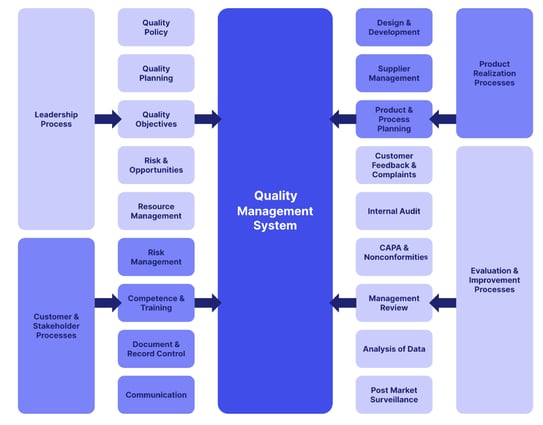
Personnel
Ensuring that personnel are trained and qualified to handle and distribute medicinal products safely and in compliance with regulations.
Premises & equipment
Setting standards for the storage and transportation facilities, including temperature control and hygiene requirements.
Documentation
Maintaining accurate and complete records of all distribution activities, including handling of complaints and product recalls.
RELATED READING: Why your life science business needs electronic document management
Operations
Outlining procedures for receiving, handling, and dispatching medicinal products, including quality control and security measures.
Complaints, returns, suspected falsified medicinal products, and medicinal product recalls
Guidelines for handling customer complaints, product returns and the recall of potentially unsafe products.
RELATED READING: How to build a complaint management system
Outsourced activities
Ensuring that contracted third-party services (e.g., transporters) meet the same GDP standards as your own operation.
DOWNLOAD GUIDE: How to manage your life science suppliers like a pro
Self-inspections
Performing regular self-inspections and internal quality audits to verify compliance with GDP guidelines and taking corrective actions when necessary.
Transportation
Specifying requirements for the transportation of medicinal products, including temperature control and security measures.
Specific provisions for brokers
Addressing the responsibilities and regulations for pharmaceutical brokers in the supply chain.
Brokers are intermediaries in the pharmaceutical supply chain who facilitate the purchase and sale of medicinal products but do not take physical possession of these products.
Instead, they connect buyers and sellers, negotiate contracts and assist with other aspects of product distribution. Chapter 10 addresses the regulatory requirements and responsibilities that apply to these parties.
Good Distribution Practice compliance
GDP compliance takes time, energy and consistent maintenance.
A recurring theme at the PDA/FDA Joint Regulatory Conference 2023 was the significance of the broader pharmaceutical supply chain.
FDA data suggests that a myopic focus on cGMP and manufacturing has caused a blind spot for other issues like contamination, adulteration and environmental control - with these problems topping the list of warning letter citation triggers for the 2015-23 period.
In this context, airtight good distribution practice and GDP compliance seem especially significant for the industry.
Here's how to get long-term GDP compliance in place.
Understanding regulatory requirements
Like any quality benchmark, your regulatory requirements are a useful starting point to show you the bare minimum expected in your territory of operation.
Good distribution practice expectations will look slightly different depending on where exactly your business is based.
Key international GDP guidelines for pharmaceutical businesses to brush up on include:
In 2023, PIC/S also published a couple of handy extra documents on their website:
1) An aide memoire for GDP supply chain inspection best practice
2) A Q&A supplement to the GDP guide
If you're in the UK, the MHRA's 2022 Green Guide maps out your GDP compliance expectations.
If you're in the EU, the EC GDP chapters will apply to you.
And if you're based in the US, take a look at the United States Pharmacopeia chapters 1079 & 1083. They aren't mandatory but offer a best practice model of good distribution practice in the US, where GDP compliance is less emphasized in regulatory documents.
The 2013 Drug Supply Chain Security Act (DSCSA) was also supplemented in 2022 with a string of new FDA documents. A 'National Standards for the Licensure of Wholesale Drug Distributors & Third-Party Logistics Providers' document was floated, but the FDA has gone quiet on the topic since.
This is one to keep an eye on.
Steps to implement good distribution practice
Begin by pinpointing your exact requirements as above.
Then map your supply chain thoroughly, assessing the level of risk for every step and handoff point: product, packaging, sites, facilities, transport, temperature controls and so on.
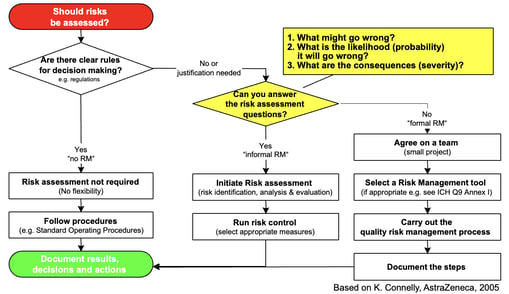
ICH Q9 offers a quality risk management model that's perfect for splicing into your supply chain and distribution activity.
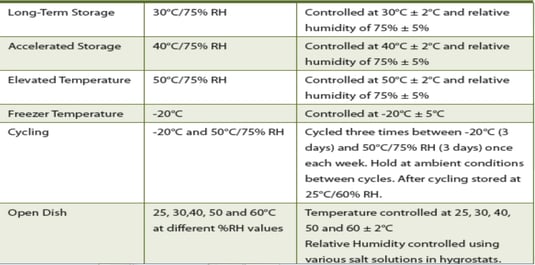
ICH Q9 emphasizes measured and commensurate response to risks: your product failure points, for instance, should determine the level of control needed to distribute that drug safely as follows:
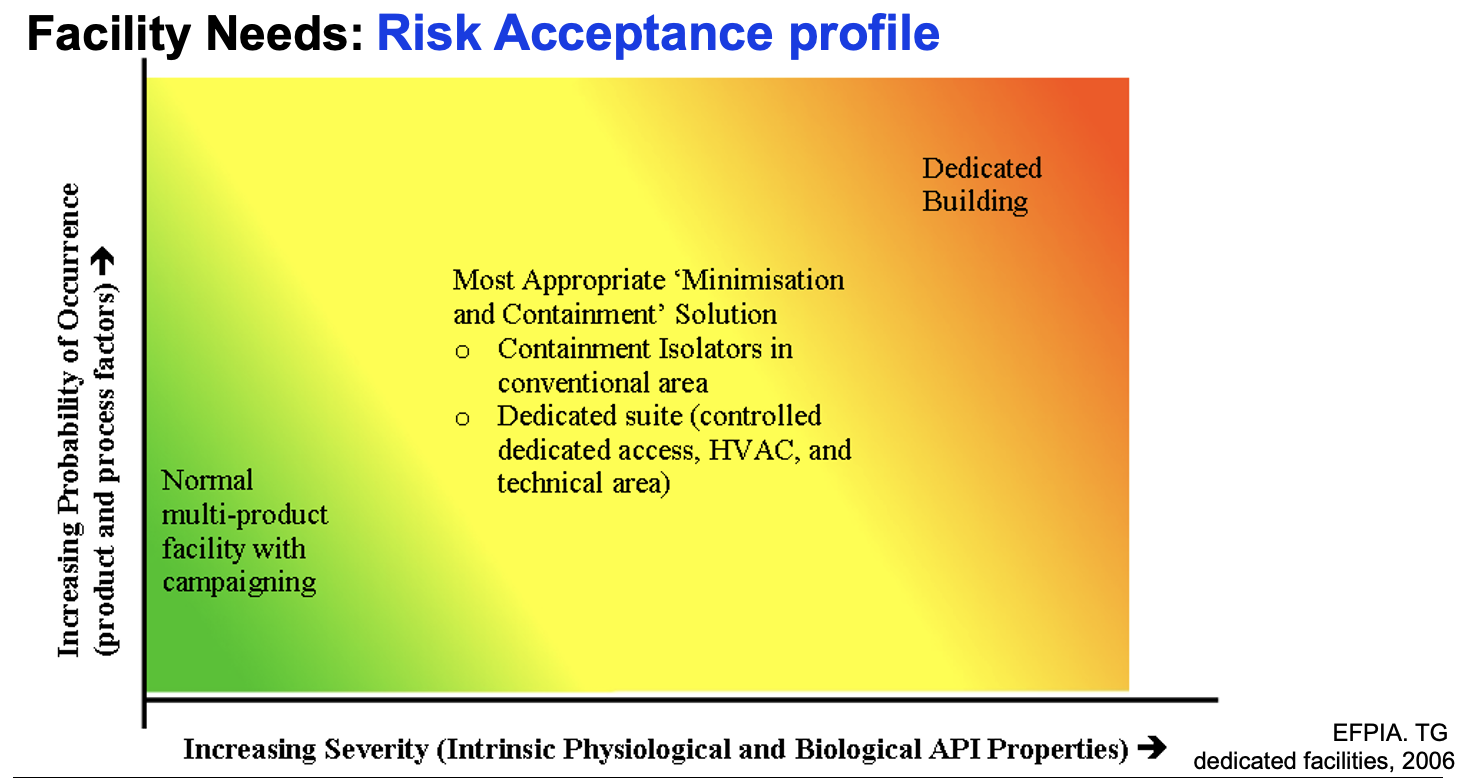
Your facility designs, too, need to take your identified risks into account:
Once you've found and documented your risks and their appropriate treatments, it's time to establish and maintain a robust quality management system (QMS) that outlines the processes, procedures and responsibilities related to good distribution practice.
This should include clear documentation of your quality policies and objectives for key GDP compliance areas.
SOPs should be made for key areas like:
- Training
- Premises and storage facilities
- Temperature/humidity control
- Risk management
- Picking and packing
- Packaging and labeling
- Security and anti-counterfeiting
- Returns and recalls
- CAPA
- Monitoring and auditing
- Outsourcing and service agreements
- Validation of computerized systems like temperature sensors, ERPs, HVACs and so on
Once you've built your bedrock of identified risks, appropriate treatments and fully documented SOPs, it's time to build a cross-functional good distribution practice team to cascade your process requirements into your operation...
Building a good distribution practice team
Key GDP compliance roles and responsibilities include:
- Quality assurance (QA) manager: in charge of quality control, documentation and compliance monitoring
- Logistics & distribution manager: manages the day-to-day logistics and distribution operations, including storage and transportation
- Risk management specialist: identifies and mitigates potential risks in the supply chain map, both at the beginning of your GDP compliance planning journey and periodically into the future
- Warehouse supervisors: oversee the proper storage and handling of products on the ground
- Quality control inspectors: conduct inspections of warehouses, trucks and other supply chain linkages to verify that robust good distribution practice is in place
A cross-functional approach to GDP compliance is particularly valuable during the supply chain mapping and risk assessment phase: encourage input from multiple facets of the business to ensure all risks are scored and treated.
Use training and internal communications to create a culture within the team that values and prioritizes compliance with good distribution practice. Encourage team members to be proactive in identifying and addressing GDP compliance issues and risks, and reward them for doing so.
Above all, remember to include senior management. Management commitment and periodic management review of your good distribution practice activity is an essential aspect of GDP compliance.
Technology and good distribution practice
Like any modern life science quality benchmark, good distribution practice is much easier to achieve with targeted application of appropriate technology.
With the right planning and investment, your GDP compliance burden can be eased and robust, automatic good distribution practice knitted seamlessly into your operation for the long term.
Choosing the right tools for Good Distribution Practice
Modern good distribution practice is almost impossible without investment in the latest tools and technology.
Temperature sensors, freezers, tracking and tracing systems, resource planning and warehouse management software, monitoring alarms and containers are all part and parcel of how modern pharma distributors operate.
Even commonplace tools like CCTV and GPS have a critical place in your supply chain.
But perhaps the most significant tool underpinning best-in-class GDP compliance is the electronic quality management system, or eQMS.
Since good distribution practice hinges on airtight control of risks within a functional and business-wide quality management system, digitizing your QMS into an eQMS is a key way to simplify and meet your GDP compliance demands.
Want an example?
Warehouse Anywhere, a national logistics expert, needed to embed good distribution practice to expand into the regulated world of life science and snag new, lucrative pharmaceutical contracts. By adopting a cloud-based eQMS and cascading digital GDP compliance across their remote workforce, the company opened millions of dollars of new revenue - securing customers like AbbVie, Eli Lilly & Co. and Johnson & Johnson.
With smart adoption of the right tools and quality mindset, robust and long-term good distribution practice is well within reach of your organization.
