What you need to know about ICH Q8
ICH Q8 (R2) is a regulatory guideline that every quality-centric pharmaceutical organization should understand, consider and implement.
ICH Q8 focuses on pharmaceutical development and the embedding of 'quality by design' into your drug's lifecycle - applied properly, ICH Q8 compliance bakes repeatable operational quality into your organization and puts quality front and center.
Here's everything you should know about ICH Q8 pharmaceutical development, and why (and how!) you should apply it to your business.
Table of Contents
Introduction to ICH Q8
ICH Q8 specifically focuses on the pharmaceutical development process, and provides guidance on the principles and concepts for developing and optimizing the formulation and manufacturing process of your pharmaceutical products.
ICH Q8 emphasizes the importance of a systematic approach to product development, by using quality risk management principles and an understanding of your product's intended use, critical quality attributes and manufacturing processes.
ICH Q8 promotes a quality-by-design (QbD) approach, which involves designing and developing your drug, and the manufacturing process it passes through, to consistently deliver desired quality attributes.
The guideline encourages the use of scientific knowledge, risk-based experimentation and careful risk assessment throughout your drug development process to optimize product quality, boost manufacturing efficiency and embed regulatory compliance.
Overview of ICH Q8
By following the principles outlined in ICH Q8, pharmaceutical companies can establish a more efficient and effective pharmaceutical development process, leading to the production of high-quality drugs that meet regulatory requirements.
Like ICH Q9 and Q10 (and unlike requirements like cGMP), ICH Q8 compliance is optional. No country or market demands adherence.
But replacing bare-minimum regulatory compliance with mature quality-by-design is worth the effort.
In 2001, a PWC report uncovered pharmaceutical scrap and rework rates touching 10%, and costs of poor quality exceeding 20%.
In 2005, an IBM report found the average pharmaceutical process had an average sigma level of 4σ. Pushing processes closer to 6σ with a robust QMS driven by continuous improvement and quality by design (QbD), the report suggested, could unlock cost savings of over $10 billion a year.
And a 2019 FDA report found overt focus on compliance, rather than quality, was holding back pharmaceutical companies and the wider industry. The FDA’s Dr. Woodcock called for the 'desired state':
‘A maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high quality drug products without extensive regulatory oversight’
Embracing ICH Q8 to unlock more advanced levels of quality management which go beyond your cGMP obligations won't only bring cGMP automatically in its wake. It helps make quality a key competitive differentiator for your business - crucial as initiatives like the FDA's quality management maturity program pick up speed.
Want your pharma business to be the best it can be? ICH Q8 is your ticket.
ICH Q8, Q9 and Q10: how they're related
ICH Q8 pharmaceutical development compliance is often tackled as part of a trifecta.
ICH Q8 (pharmaceutical development) sits alongside ICH Q9 (quality risk management) and ICH Q10 (pharmaceutical quality management) as a three-spoked model of modern pharmaceutical best practice, as suggested by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH).
ICH has used the analogy of a three-legged stool to illustrate this interacting, symbiotic relationship.
RELATED READING: What is an ICH Q10 pharmaceutical quality system?
The logic is clear: grafting quality-based drug development (ICH Q8) and risk-based thinking (ICH Q9) into a robust overarching pharmaceutical quality system (ICH Q10) gives pharmaceutical companies a clear framework for optimizing drug safety, efficacy, quality and availability.
Pharmaceutical development: ICH Q8 (R2)
The full title of the guideline, if we're being completely precise, is ICH Q8 (R2).
The 'R2' designation indicates that this is the second revision of the ICH Q8 guideline: the original underwent an update to incorporate feedback from stakeholders and to reflect industry advancements.
The aim of the game for ICH Q8, then, is robust pharmaceutical development, achieved with the implementation of quality by design (QbD).
Strategies for quality by design (QbD)
What is quality by design?
It's a systematic and science-based approach to pharmaceutical development and manufacturing that aims to maximize product quality and patient safety.
In the context of pharmaceuticals, 'QbD' involves designing and developing a product and its manufacturing process to consistently deliver your desired quality attributes.
QbD emphasizes the understanding of how various manufacturing and development factors and variables affect product quality and performance, encouraging manufacturers to proactively identify and control potential sources of variability throughout the drug lifecycle.
The core strategies for QbD include:
-
Defining the Target Product Profile (TPP): The desired quality, safety and efficacy characteristics of the pharmaceutical product, as well as its intended use (more on that below!)
-
Pinpointing Critical Quality Attributes (CQAs): The specific physical, chemical, biological or microbiological characteristics of the product critical to ensuring its quality and performance. Identified based on the TPP, and relevant scientific knowledge
-
Designing and mapping the manufacturing process: Identifying so-called 'critical material attributes' (CMAs) and 'critical process parameters' (CPPs), and their relationship to your CQAs. Process optimization and validation are performed to ensure consistent product quality.
-
Risk assessment and management: ICH Q9 is your main destination for pharmaceutical quality risk management guidance. But ICH Q8's QbD has a lot to say too, suggesting systematic risk assessment and mitigation strategies to identify and control potential sources of manufacturing variability
-
Real-time process monitoring and control: Closely related to points 3 and 4, QbD emphasizes the use of advanced process monitoring and control strategies, such as process analytical technology (PAT), to continuously monitor critical process parameters and ensure process standardization
-
Continuous improvement: Like ICH Q9 and Q10, Q8 makes it clear that continuous optimization should be your end goal. QbD is described as an iterative approach, where the knowledge gained throughout the development and manufacturing process is used to continuously tweak and improve your product quality and manufacturing efficiency
All of these threads should combine into a robust quality strategy that's proactive and quality-focused, rather than reactive and focused solely on baseline regulatory compliance:
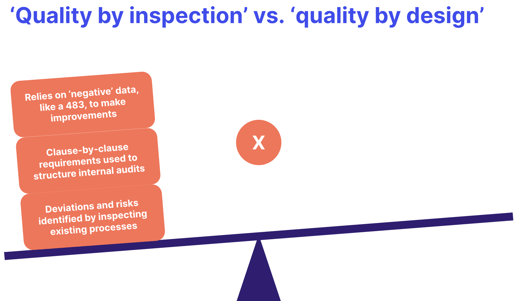
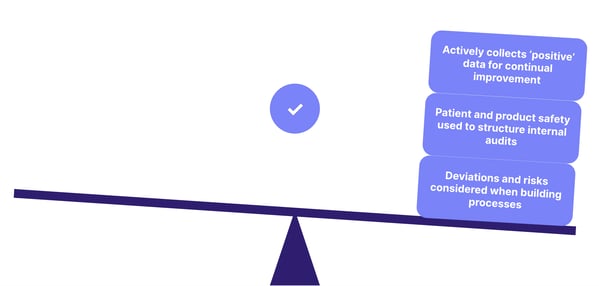
Watch our webinar recording:
Goals of pharmaceutical development
ICH Q8 pharmaceutical development was developed by the ICH with clear end goals in mind.
We touched on the FDA's 'desired state' above.
It's also worth considering the basic goals of good manufacturing practice (GMP), which the ICH took inspiration from. FDA 21 CFR Part 210 defines the key objective as being able to:
"... assure that [a] drug meets the requirements of... safety, and has the identity and strength and meets the quality and purity characteristics that it purports or is represented to possess."
These basic requirements of quality, efficacy and safety should always be kept in mind.
But ICH Q8's blueprint of pharmaceutical development allows you to take things a step further. Other goals to consider as you prepare for ICH Q8 compliance include:
-
Patient centricity: Your pharmaceutical development process should consider the needs and preferences of patients at all times. This involves developing drugs that are easy to use, convenient and acceptable to patients, thus promoting patient adherence to treatment regimens
-
Cost-effectiveness: Developing pharmaceutical products that are cost-effective is important for both patients and healthcare systems. A robust, QbD-led development framework should minimize costs without compromising product quality or safety
-
Innovation: New drug discovery, formulation and delivery systems, including new therapeutic options and technologies, should have the ability to be delivered nimbly, quickly and safely to address unmet medical needs and improve patient outcomes
-
Timeliness: Your pharmaceutical drugs should get to market in a timely and consistent manner. Optimizing your development process allows expedited timeframes without sacrificing scientific rigor and compliance, and minimizes the risk of supply chain disruption
Now we've touched on these topline goals and objectives, let's dive deeper into the specific ingredients of ICH Q8 (R2) compliance.
FURTHER READING: The key to future-proofing pharma compliance
Quality Target Product Profile (QTPP)
What is a QTPP?
It's a comprehensive description of the desired quality, safety and efficacy characteristics of your pharmaceutical drug - and a key component of your ICH Q8 pharmaceutical development planning!
Defining QTPP
ICH Q8 officially defines the QTPP as:
"A prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy."
Your QTPP is, in essence, a laundry list of what you want your pharmaceutical product to be.
Only by setting this desired end goal can you build and manage your development processes to get there.
Key elements of a QTPP may include:
- Identification of your product: dosage form, strength, route of administration, etc.
- Therapeutic attributes: effects, duration of action and therapeutic indications
- Critical quality attributes (CQAs): As we touched on above, your CQAs are the parameters that set and guarantee the safety, efficacy and quality of your product. They could include physical, chemical, biological, and microbiological properties, such as potency, purity, dissolution rate, particle size, stability, impurity profile, and so on
- Patient requirements: The QTPP may include specific patient-centric requirements, such as dosage form characteristics, packaging or taste masking.
-
Manufacturing considerations: The QTPP may specify aspects related to the manufacturing process, such as desired scale of production, control strategy and process validation requirements
Here's an example!
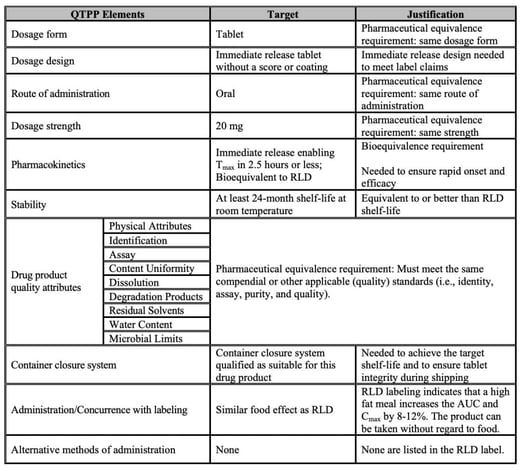
Importance of QTPP in pharmaceutical development
The QTPP should be developed early in your ICH Q8 pharmaceutical development process, since it serves as a reference and target point for the entire product lifecycle.
It provides a clear understanding of your target product and helps guide decision-making throughout your pharmaceutical development, manufacturing and quality control/assurance activities.
Formulation development, process optimization, analytical method development, manufacturing process scale-up and so on should all be aligned with the end goals documented in your QTPP.
Critical quality attributes (CQAs)
Since quality by design hinges on finding and controlling the factors impacting your product quality, critical quality attributes are the fixed benchmarks your product should consistently hit.
By identifying and prioritizing your CQAs, effort and energy can be directed at the critical aspects of your drug development and manufacturing, to ensure that these attributes are consistently met throughout your product lifecycle.
ICH Q8 defines the CQA as:
"A physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality."
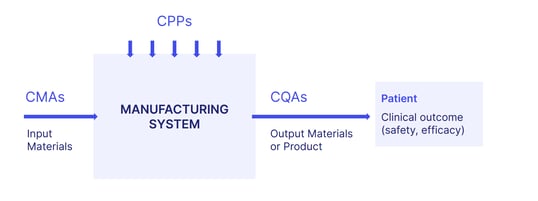
Identifying CQAs
Your CQAs will vary depending on your specific product and its dosage form.
They may include attributes such as drug potency, purity, dissolution rate, particle size distribution, content uniformity, stability, impurity profile, bioavailability, sterility or endotoxin levels.
To find a CQA, ask yourself these 4 questions:
1) "Does this characteristic impact the quality of my product?"
Remember that your CQAs should have a direct correlation with the safety, efficacy or quality of your pharmaceutical product, affecting performance, stability and/or functionality.
2) "Can I measure it?"
CQAs should be measurable using appropriate analytical methods or tests, so they can be constantly assessed.
3) "Can I control it?"
The old adage of 'it's only a problem if you have a solution' can be applied here.
CQAs should be able to be controlled or influenced by formulation, manufacturing process or other critical factors.
4) "Is it relevant?"
Not every attribute of your product is relevant to your regulatory compliance. The color or odor of your drug might have no bearing on the safety and efficacy requirements in a specific territory you want to sell your drug in - but its dissolution rate certainly will.
Role of CQAs in ensuring product quality
By finding your CQAs then measuring and controlling them, your ICH Q8 pharmaceutical development strategy will be bolstered by appropriate product specification benchmarks, which in turn help you implement effective control strategies that ensure consistent product quality.
CQAs also allow for risk assessment and mitigation to prevent or manage potential variability that could impact product performance or patient safety.
CQAs are therefore a key area of crossover between ICH Q8 and Q9, allowing you to assess and control risks appropriately with product quality as your guiding light.
This brings us nicely to...
Risk assessment and design space
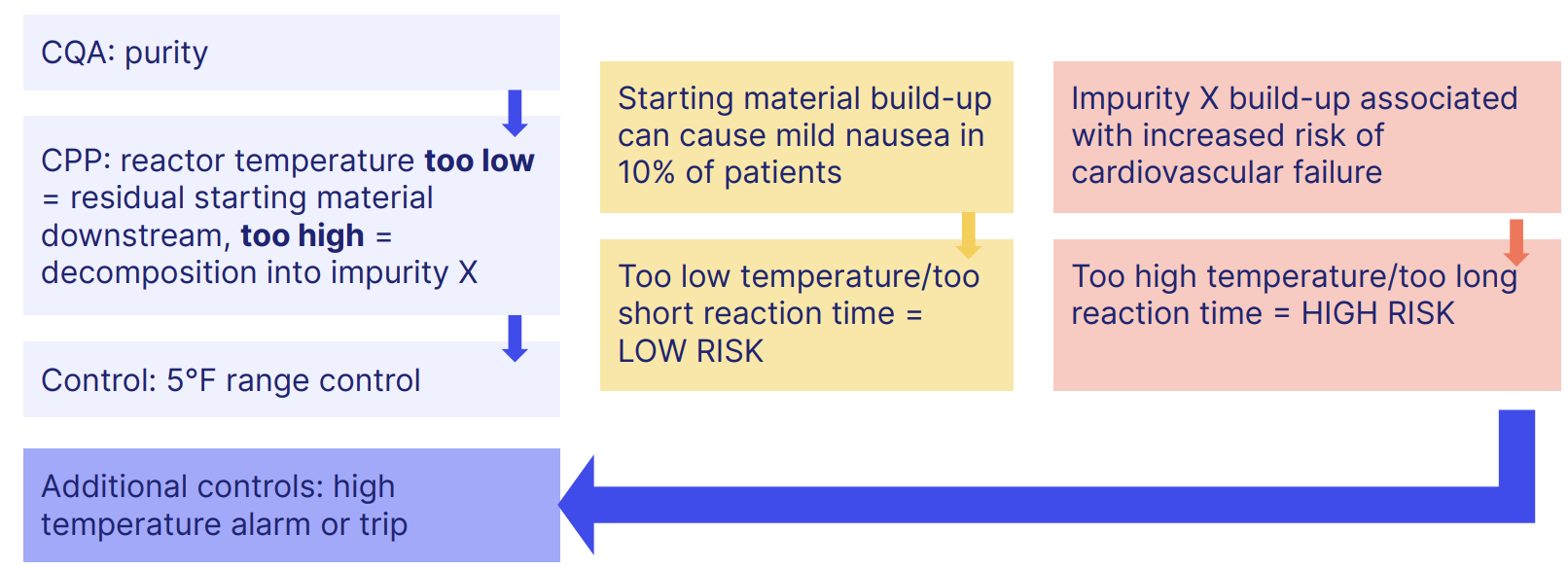
Now we have our CQAs, ICH Q8 risk assessment focuses on identifying which material attributes and critical process parameters will impact them.
Here we can see how the CQA of 'purity' interacts with critical process parameters to structure an entire risk strategy, culminating in a specific set of risk controls that keep a specific product quality benchmark in check.
Risk assessment methods
Risk assessment within ICH Q8 hinges on finding and documenting the risk factors and variables with potential impact on product quality.
One useful method is the completion of an Ishikawa, or fishbone, diagram:
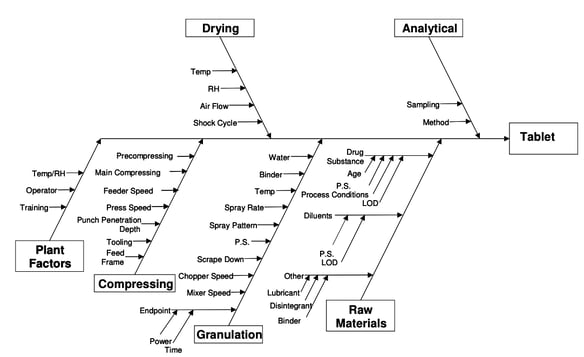
Source: ICH Q8 (R2)
FMEA analysis could then be applied to each identified variable to rank them by severity, probability and detectability - with appropriate risk controls then applied and tested.
FURTHER READING:
Establishing the design space
In ICH Q8, the design space is defined as the physical combination and interaction of input variables that assure your product quality.
It's the range of conditions (such as formulation components or process parameters) within which your pharmaceutical product should be developed and manufactured to keep its desired quality attributes.
Your design space should take into account the relationship between the CPPs and CQAs of your product to find an optimal inflection point.
Think of your design space as the bullseye of a target, or the spot on your car's speedometer to keep your needle so you don't get a speeding ticket or hold up the guy honking behind you.
It's where you want all your process variables to 'land' so that your outputted drug is safe, effective and made as desired.
ICH Q8 offers some helpful examples in its Appendix II to show you how your design space could be conceived of and illustrated.
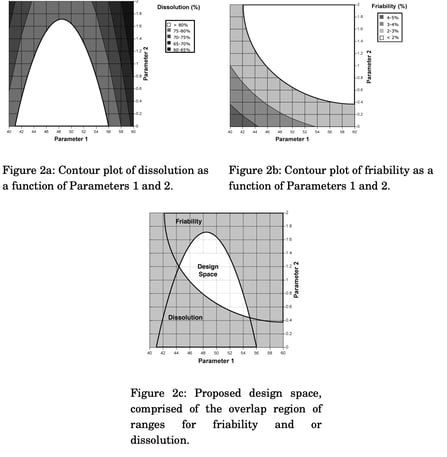
Source: ICH Q8 (R2)
Process analytical technology (PAT) in ICH Q8
How do we measure all this stuff?
As you've no doubt realised by now, ICH Q8 pharmaceutical development encourages the use of analytical techniques, real-time process monitoring and clear, measurable control strategies.
You can't do all that by just sitting and watching your drugs being made, ticking things off on a paper checklist.
It's for this reason that process analytical technology, or PAT, goes hand-in-hand with ICH Q8 compliance.
ICH Q8 itself defines PAT as:
"A system for designing, analyzing and controlling manufacturing through timely measurements (i.e., during processing) of critical quality and performance attributes of raw and in-process materials and processes with the goal of ensuring final product quality."
With everything we've covered, you can see how PAT simplifies and enables adherence to ICH Q8's principles.
ICH Q8's Appendix I compares 'minimal' pharmaceutical development - empirical checks, one-at-a-time variable research, in-process tests - with 'enhanced' QbD methodology, where PAT tools unlock systematic, fixed design spaces underpinned by multivariate experiments and robust tracking and trending.
Implementation of PAT tools
How do you get PAT tools onboard, then?
There are several steps you should follow to ensure smooth and successful PAT integration into your development and manufacturing processes.
-
Define your objectives: Identify the specific areas, processes or products to which PAT will be applied, and the expected benefits
-
Map your processes: Determine the key parameters and quality attributes that need to be monitored and controlled with PAT technology
-
Develop a PAT team: As you would with any major system investment, build a multidisciplinary team that includes experts from various departments connected to the new system. Get input from formulation, manufacturing, analytical and quality teams!
-
Assess the market: PAT tools can include spectroscopic techniques, chromatography, imaging systems, and other real-time monitoring and analysis technologies. Be clear of what exactly you need, how far your budget can stretch, and what you can include or discount in your search
Done properly, your PAT should form a positive feedback loop, deepening your visibility of key processes and then giving you the data to drive measurable continuous improvement.
We saw how the design space is your 'sweet spot' development and manufacturing target. Choose the PAT that lets you define, measure and hit that spot consistently.
Control strategy
As ICH Q8 puts it, you'll need an effective control strategy to 'ensure that a product of required quality will be produced consistently'.
That means being able to describe and justify how the execution of in-process controls, input and intermediate controls and your container closure system all contribute to your final product quality.
Developing a control strategy
A clear control strategy is essential for ICH Q8 pharmaceutical development.
It's the planned set of controls, derived from your process and product understanding, that ensures the desired quality of your pharmaceutical product is consistently achieved.
As you develop yours, it should include:
- Product specs
- Control of input material attributes (drug substance, excipients, primary packaging materials and so on) based on an understanding of their quality impact
- Control of operations that have an impact on downstream product quality
- In-process or real-time release testing (preferably with PAT!)
- A monitoring program with verification of multivariate prediction models
Ongoing process verification
Like ICH Q9 and ICH Q10, ICH Q8 encourages continuous improvement and optimization.
Your analytical and control strategy should therefore have a mechanism for ongoing verification, measurement and improvement built in.
This should involve periodic reviews, trending analysis and repeatable risk assessments to identify potential areas for improvement or process modifications.
Future developments in pharmaceutical quality by design
Quality by design, by nature, can't be a static end goal that you hit and rest on forever.
It must be proactive, future-looking and continuously maturing and improving.
Industry initiatives like the FDA's quality management maturity program show a clear appetite for promoting and rewarding quality-focused pharmaceutical operations.
Pharmaceutical winners in the next decade will be those that embrace ICH's triple model of Q8, Q9 and Q10 as a springboard for real quality centricity.
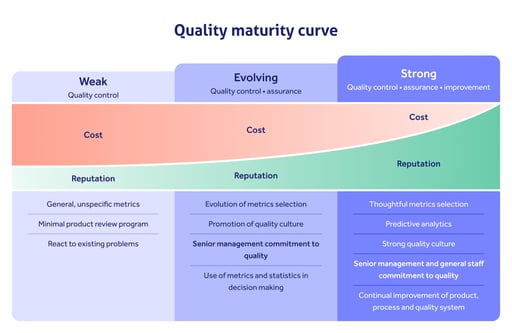
Alongside critical pharmaceutical development tools like PAT, consider how to strengthen your quality management activity with tools like pharmaceutical quality management software.
Organizations that digitize key quality activity like document, training, event and supplier management, while keeping on top of the latest industry expectations and going beyond baseline cGMP, will be those that position themselves for maximum success as regulators push pharmaceutical quality to an unprecedented degree.
ICH Q8 pharmaceutical development is your roadmap to industry-leading, competition-beating quality focus. Put it to good use.
