Introduction to quality control software
Whether you're in a pharmaceutical lab, a biotech company or a medical device manufacturer, quality control is a fundamental compliance obligation for modern life science companies.
And it's here that quality control software comes in as a critical tool.
Thinking of optimizing your quality and compliance activities with purpose-built quality control software? You're in the right place. We’ll explore what quality control software is, how it supports life science companies like yours, and how to choose the best system.
What is quality control software?
Quality control software, usually offered as part of a broader electronic quality management system (eQMS) software platform, is any digital software designed to help regulated organizations manage the processes, data, documentation and workflows involved in modern quality control.
It transforms what used to be manual and analog QC tools, like paper logs, spreadsheets and checklists, into digital, auditable, flexible automated workflows.
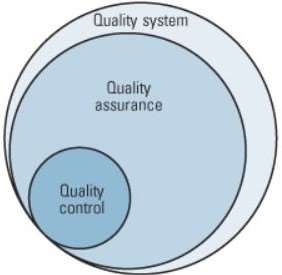
Quality control sits within the bundle of critical activities that form the modern quality management system, so it has to be done properly.
At its core, quality control software typically handles:
-
Document management: handling the end-to-end lifecycle of key quality documents like standard operating procedures (SOPs), work instructions and quality manuals while ensuring correct versioning, integrity & access control
-
Training management: recording that staff have read and understood relevant procedures and are qualified to perform their roles.
-
Event/nonconformance/CAPA management: logging defects and deviations, investigating causes, triggering corrective and preventive actions, and tracking follow-up.
-
Change control and audit readiness: ensuring every process or document change is controlled, tracked, and compliant for regulatory audits.
-
Supplier & vendor management: managing supplier qualification, performance and overall supply chain quality.
In bringing these activities together, quality control software helps organizations ensure that their products, from lab test results to completed drug batches, consistently meet predefined quality and regulatory standards like FDA 21 CFR Parts 210 and 211, ISO 13485 or ICH Q10.

Transitioning from manual, paper-based systems to digital quality control software becomes especially critical as companies scale. For small operations, spreadsheets and paper can seem manageable, but as complexity increases, manual QC systems become error-prone and hard to audit.
FURTHER READING: Quality management vs assurance vs control
Quality control software for manufacturing (and why it matters for life science)
Quality control software for manufacturing tends to be more appropriate for companies making highly regulated products rather than, say, toys or furniture.
In the context of life sciences, manufacturing might involve:
-
Production of pharmaceuticals or biologics
-
Manufacturing of medical devices or diagnostic kits
-
Contract manufacturing or outsourcing (CMOs/CDMOs) producing drugs or biologics for other companies
For these highly regulated operations with high compliance burden, QC software helps your team ensure that every product is made under controlled, compliant and fully traceable conditions.
What does quality control software bring to life science manufacturing?
-
Audit readiness and compliance: Quality control software helps ensure that documentation, processes, traceability and controls are in place and ready before your auditor or inspector walks through the door.
-
Quality-driven culture: By uniting tasks like document control, training records, CAPAs, supplier management and risk management in one platform, quality control software helps build a culture where quality isn’t a side-project but embedded into every activity. Users coordinate and collaborate to close out quality control tasks together with end-to-end visibility.
In short: quality control software for manufacturing ensures that life science companies don’t just produce their critical products, but produce them reliably, safely and compliantly. It replaces fragmented, manual procedures with a single, controlled and consistent digital quality control backbone.
FURTHER READING: 2026 guide to pharmaceutical software
Laboratory quality control software: enabling QC and compliance in labs
Production manufacturing isn’t the only facet of life science that typically requires investment in quality control software.
Laboratories, from clinical testing to diagnostics, can also rely heavily on quality control software to maintain integrity, traceability and regulatory compliance.
Key use cases include:
- Instrument and equipment management: Many quality control software solutions integrate with lab instruments (e.g. chromatographs, microscopes, bio-analyzers) to automatically capture data, reducing manual data entry and human error while maximizing data integrity.
- Workflow automation: From test planning to execution, result capture, quality control checks and sample review, laboratory quality control software allows users to build flexible, collaborative and repeatable workflows that ensure consistency across lab operations.
- QC result tracking and alerts: Software can monitor QC control charts, flag out-of-spec results, and trigger alerts for corrective action: critical for meeting laboratory quality standards for purity, potency, contamination and stability.
-
Compliance & audit trailing: Labs operating under standards like ISO 17025 and ISO 15189 need to demonstrate an integrated laboratory quality management system underpinned by robust documentation and audit trails. Quality control software supports this, while also providing electronic record and signature compliance for regulations like FDA 21 CFR Part 11 and EU Annex 11.
-
Reporting & traceability: By centralizing data, quality control software simplifies generation of key outputs like audit reports and deviation reports while ensuring full traceability of decisions and results.
In life science labs, quality control software therefore dramatically reduces risk, manual workload and compliance burden, while optimizing the reliability and reproducibility of test results.
Best quality control software for life science
If you're evaluating quality control software for a life science company, the best system for you depends on several factors: your regulatory compliance needs, your size and complexity, your existing systems, and your scalability and integration requirements.
That said, there are a few solutions widely used in the life science industry because they are purpose-built for its regulatory and operational needs.
What to look for in life science quality control software
When choosing a software solution, consider:
-
Compliance support: Does the platform support regulatory requirements relevant to your industry? In other words, is it purpose-built for life science?
-
Validated system with audit readiness: For regulated environments, the software should be validated — ideally in accordance with the latest CSA best practice. It should also provide compliant electronic signatures, audit trails and data integrity features as standard.
-
Integrated functionality: A good quality control software system should be comprehensive enough to cover all modern QC requirements (document control, CAPAs, training, change control, audit, supplier management, and so on) so you don’t need to rely on multiple fragmented tools.
-
Ease of use and scalability: The software should be flexible and easy to use so it can scale with you, ideally in a cloud-based environment.
-
Integrations: Modern life science companies rely on a stack of tools, from CRMs and design/test systems to LMS platforms. Prioritize quality control software systems that integrate with these other business-critical tools to provide a connective 'quality layer'.
Top life science quality control software systems
-
SimplerQMS: A cloud-based QMS built specifically for life science companies. Supports compliance with key standards and regulations like GxP, ISO 13485, FDA 21 CFR Part 11/820/211, the EU MDR/IVDR and more. Modules include document control, CAPA, equipment management, training and audits.
-
Qualio: A holistic quality and compliance platform purpose-built for life science companies, and used by over 700. Integrates all your quality management processes in a holistic eQMS, but also offers Compliance Intelligence: a unique, AI-powered intelligence layer that scans your entire quality system, surfaces compliance gaps for your standards and regulations, then guides you to close-out for constant audit readiness.

-
Scilife: Another life science-specific quality platform. Scores highly for ease of use and offers a good suite of functionality, but lacks the integrations, pre-built life science content and killer AI features of Qualio
-
Dot Compliance: A flexible, scalable system that's nice to use with some nifty life science functionality like pharmacovigilance and regulatory submission modules, but lacks design controls for medical device operations.
Why generic quality control software isn’t enough: the case for purpose-built
Why not just use a generic, industry-agnostic, cheaper quality software system?
It's a tempting proposition for small organizations and early-stage ventures.
But it's a bad idea.
Generic quality control software solutions tend to fall short in life science operations, and here's why:
-
Regulatory requirements: Regulators demand strict quality and compliance data traceability, backed by audit trails, properly validated systems, electronic signatures and ALCOA+ data integrity. Generic quality control tools often lack these features out of the box.
-
Integration across disciplines: Larger life science organizations can involve an interacting mixture of lab work, manufacturing, supply chain management and audit prep activities. A purpose-built industry tool unites these tasks, breaking down any silos that could undermine oversight and increase risk.
-
Scalability and validation needs: As your organization grows — more products, more batches, more tests, more people — quality and compliance burden magnifies. Generic quality tools scale poorly and often require manual workarounds and additional systems to keep compliance on an even keel.
-
Risk and CAPA management: When deviations occur, like a contaminated batch, a failed QC test or a supplier nonconformance, life science companies must properly document, investigate and correct them to prevent recurrence. Most reputable purpose-built life science quality control software systems provide CAPA workflows as standard, giving you the ability to execute fully digitized root cause analysis, remediation and documentation.
- Pre-built content: Operating in an industry niche naturally unlocks more targeted, valuable support from quality control software vendors. Qualio, for instance, offers pre-built content and templates for its life science customers, ranging from medical device QMS ingredients to pharmaceutical SOP and quality manual templates.
While generic quality tools like agnostic eQMS systems, paper and spreadsheets may seem cheaper or easier at first, in a regulated, high-risk industry like life sciences they usually represent false economy.
The cost of non-compliance is simply too high for life science operations, making purpose-built tools the way to go.
Challenges and considerations when implementing life science quality control software
Adopting life science quality control software is not without its challenges.
Here are some to watch out for, along with best practices to overcome them.
Challenge: validation & compliance requirements
Solution: Choose a vendor with specialized validation support, ideally based around the latest CSA best practice. Look for a GAMP 5 Category 4 system for the best balance between flexibility, configurability and validation demands.
Challenge: Buy-in and cultural shift
Solution: Dedicate time for training, define clear responsibilities, and communicate the benefits of moving to quality control software: less manual work, more time for value-add initiatives, no more audit scramble stress).
Challenge: Integration with existing tech stack
Solution: Choose a platform with good integration capabilities and APIs, plan your architecture early and map out which processes need to be connected.
Challenge: upfront cost and resource commitment
Solution: Treat quality control software as what it is: an investment rather than a cost. The reduction in manual work, quicker audits, and faster time to market unlock long-term savings.
FURTHER READING: How a Qualio customer unlocked 5x ROI in 2 months
Make the right investment in quality control software
Quality control software is no longer just a tool or a convenience.
For life science companies, it represents the foundation of your quality and compliance requirements. By replacing fragmented, manual and paper-based quality control practices with integrated, auditable and automated digital workflows, quality control software puts quality front and center of your operation.
Need to make the jump to a stronger quality approach?
You're in the right place.

