Quality assurance vs. quality control explained: 5 key differences
What is quality assurance vs. quality control?
Modern quality management is a complex discipline, covering a range of activities. And in regulated sectors like life science, processes like quality assurance (QA) and quality control (QC) are both required by standards like FDA 21 CFR 820 or ISO 9001.
What is quality assurance? How does it differ from quality control? And how can you embed both?
Find out with this comprehensive blog post guide.
QA vs QC
Quality assurance and quality control are often used interchangeably, but they're two distinct processes taking place at different times. Each plays an individual, complementary role that's vital for an effective and holistic quality management system.
Understanding and applying both quality control and quality assurance activities will help your organization's operational quality be the best it can be.
To help you, this blog post reviews the QA vs QC breakdown, looks at the meanings of both activities, and then explores some quality assurance vs quality control examples to bring the difference to life.
What is quality assurance?
Quality assurance is a critical component of a quality management system that's applied in a wide array of regulated industries, like pharmaceuticals and medical devices.
Quality assurance is the bundle of activities that ensures the planned characteristics of a product (its strength, efficacy, build, ingredients, dimensions, and so on) or of a service (response times, results, project timelines) are achieved in reality. The company making the product or delivering the service can therefore be sure that it's done what it intended to do, and satisfied the requirements of its customer.
Quality assurance, then, plays a critical role in ensuring that the potentially life-saving products making their way to patients perform as they should.
You may also sometimes hear quality professionals refer to the '4 types of quality assurance'.
What are the 4 types of quality assurance?
There isn't a standardized industry definition, but the 4 groups of process, product, organizational and supplier quality assurance are a common and useful categorization.
As the names suggest, quality assurance is typically directed towards each of these areas: your organization, the processes that flow through it, the products you make, and the suppliers you interact with.

Quality assurance definition
The general quality assurance definition offered in the US Code of Federal Regulations (23 CFR § 637.203) summarizes quality assurance as, "...all those planned and systematic actions necessary to provide confidence that a product or service will satisfy given requirements for quality."
In order for it to be effective, quality assurance means putting a formalized system in place by means of plans, policies and processes to assure that your products are manufactured accurately and in compliance with your relevant standards.
As a specific example, an FDA-regulated manufacturer’s quality management system (QMS) would be required to be in line with respective current good manufacturing practice (cGMP) requirements.
A quality assurance program would therefore be established to guarantee that outputted products align with those regulatory demands.
In addition to simply complying with their relevant regulations, as companies grow and evolve they may also impose tighter standards and quality objectives to improve customer satisfaction and overall product quality and safety.
Quality assurance could therefore be applied to ensure that internal quality benchmarks, such as a certain defect percentage or deviation rate, are hit and maintained.
In short: think of quality assurance as the act of 'assuring' or 'ensuring' that your products and services meet their quality requirements.
FURTHER READING: The ultimate guide to pharmaceutical quality assurance
What is QA?
Quality management is a complex industry full of acronyms: GxP, ISO, FDA, NCR, and so on.
QA is simply one of the many acronyms to learn in the quality world!
What does QA stand for? Quality assurance, of course.
Quality assurance is often shortened to QA, and quality control to QC, giving us the QA vs QC dichotomy.
QA is, then, just a useful shorthand to refer to the quality assurance process. It's for this reason that you'll often see industry professionals with snappy, abbreviated titles like 'QA Manager' or 'QA Associate'.
With all this in mind, what does applying QA to your business actually mean in practice?
Quality assurance meaning
Quality assurance, or QA for short, means establishing a repeatable, measurable and proactive process into your manufacturing lifecycle that's aimed at preventing defects, errors and deviations from occurring, rather than just identifying and fixing them after they have already occurred.
The meaning of quality assurance is to establish and maintain a particular level of quality throughout the entire lifecycle of your product and/or services.
This involves defining your applicable quality standards, setting up processes and procedures to meet those standards, then monitoring and evaluating the results to ensure compliance.
One well-known quality assurance framework is derived from ISO.
ISO 9001:2015 is the most widely recognized international standard used for quality assurance. Other industry-specific standards, like ISO 13485 for medical devices, all derive from the general ISO 9001 quality assurance roadmap.
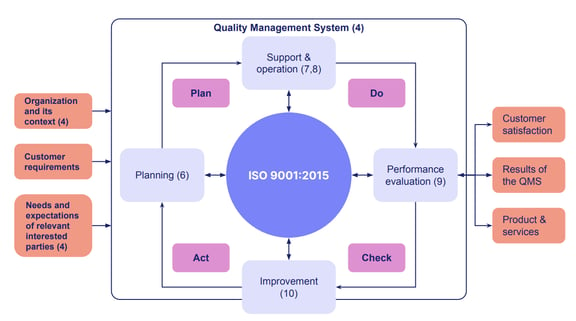
Total Quality Management (TQM) is another method, whereby your entire company is trained and involved in improving processes, products, services and the culture in which they work. TQM is customer-focused, and all employees are empowered to make continual improvement in the system for the betterment of the customer.
There are many more. These are just the most well-known, but they all have an end goal of maximizing customer satisfaction by manufacturing products that meet required standards.
FURTHER READING: The top 10 life science quality assurance consultants to work with
The quality assurance process
Quality assurance typically means having several key activities in place, including:
1. Planning
Defining quality objectives, requirements, and standards for the project or process
2. Design and development
Ensuring that appropriate quality controls are implemented during the design and development phases to prevent issues and errors
3. Process implementation
Implementing processes, procedures, and guidelines to ensure consistent quality in the production or delivery of products or services
4. Inspection and testing
Conducting inspections, tests, and evaluations to verify that products or services meet the defined quality standards and requirements
5. Continuous improvement
Identifying areas for improvement based on feedback, data analysis and customer satisfaction metrics, then implementing corrective and preventive actions to enhance quality and prevent future issues
6. Documentation and reporting
Maintaining detailed records of quality-related activities, including test results, corrective actions and audit findings, then reporting them to relevant stakeholders for their input
Regulated companies must perform regular QA inspections and tests to ensure they're adhering to their quality standards.
Quality assurance also involves corrective action when deviations or errors occur, so that the product is still safe and effective.
Overall, quality assurance requires a combination of planning, testing and monitoring. Companies must develop strategies for meeting quality standards before any product is manufactured. Testing activities should also be performed during the production process and validated at the end. Finally, monitoring should take place over time to ensure that quality standards are maintained.
Effective QA gives you a competitive edge by making sure your products meet stringent regulatory requirements. Quality assurance helps companies avoid costly recalls and maintain a good reputation.
Quality assurance should, therefore, be a key part of your quality management plan.
FURTHER READING: How to develop and implement a quality management system (QMS)
What is quality control?
We've covered quality assurance - so what is quality control?
Quality control is a series of test procedures used to verify that your product is safe and effective after production, and before it's sent to the customer.
Where quality assurance involves the broad bundle of systems and processes that interact to ensure quality products, quality control is the targeted, nuts-and-bolts 'check-up' of products as they're being produced to ensure that QA plans are corresponding to the reality of your manufacturing output.
QC, then, is all about the inspection, measurement and testing of your products and processes to detect and correct deviations from desired quality levels.
Quality control definition
The typical quality control definition is that it's the inspection 'phase' or 'subpart' within the broader scope of your quality assurance activity.
23 CFR § 637.203 summarizes it as, "all contractor/vendor operational techniques and activities that are performed or conducted to fulfill the contract requirements".
Quality control is the most basic, fundamental quality management activity, and the act of checking finished product to ensure it's up to scratch is often the first thing people imagine when they think of the quality discipline.
Without this control, there's no way for organizations to ensure their quality plans translate into reality and that contractual arrangements are being met. Product defects wouldn't be discovered until products reach the customer, prompting returns, complaints, and damage to reputation.

Quality control meaning
If QA is the overall quality 'plan', QC is how you ensure the plan is actually working at the coalface.
In this context, 'controlling' quality means evaluating it, ensuring it hasn't slipped, and discarding or reworking any finished products which aren't up to the mark.
In a pharmaceutical context, this could mean testing a completed drug batch. If your critical quality attributes aren't achieved, the batch is discarded and a new one created.
With this in mind, let's look closer at the quality assurance vs control breakdown.
Quality control vs quality assurance
A common misconception is that quality assurance and quality control are exactly the same thing: 'quality'.
But while they might be under the same umbrella, they are operationally different.
It's therefore important to understand the key quality assurance vs quality control differences.
As we've seen, quality control is the set of steps put into place to check the compliance and accuracy of a product (or service) after it is produced.
One way to understand the contrast is to think of quality assurance as a 'proactive' or 'preventative' process, with quality control as the 'reactive' process put in place for reviewing and checking products after they are manufactured to ensure no defects or faults have emerged.
So for quality assurance: think processes put in place to assure quality before the fact.
For quality control: think 'inspection' steps after the fact, to check and 'control' the product and resolve any issues before it reaches the customer.
Both are important for ensuring the end quality of your product and service, but come into play at two different points in the manufacturing process.
And in the totality of your quality management system, quality control is often considered a subset of your overarching quality assurance measures.

Source: ASQ
As you can see in the diagram, both QA and QC are necessary: the quality control vs assurance binary isn't a 'one or the other' approach, because you can’t test quality into a product by relying on QC alone.
There are different methodologies for both QA and QC, like Agile, Six Sigma, and other project management or process improvement strategies. They’re designed to save time while managing your quality and project development.
Using these can help, but you’ll need that overarching quality management system to effectively manage the entire quality planning process.
Let's look at some examples for quality control vs assurance, and how the two differ in reality.
Quality assurance vs. quality control examples
To further understand the differences between quality assurance and quality control and ensure you can embed both in harmony, you need to get a picture of how the two processes work.
1. Proactive (QA) vs. reactive (QC)
A key QA vs QC difference is proactivity.
Effective quality assurance is proactive. It aims to prevent defects before they occur through process design.
QC is reactive and exists to identify defects in the quality of products after they have happened.
QA involves the design of processes, such as documenting standard operating procedures (SOPs) according to relevant industry standards. A safe, effective product should be the result every time processes are followed.
QC, meanwhile, involves the testing of products to ensure they meet those standards for safety and efficacy. If QC testing uncovers quality issues, it should result in reactive steps to prevent an unsafe product from being shipped and distributed.
Ideally, QC issues should also spark a QA review.
In a classic quality assurance vs quality control example, a non-conforming test result, like a medical device which exceeds error tolerance levels, would be a QC output, and should result in a QA corrective and preventive action (CAPA) investigation to determine the root cause of the issue and update processes to prevent the problem from happening in the future.
2. Process (QA) vs. product (QC)
Another difference between QC and QA is around the target of the activity.
QA is process-oriented, and it focuses on preventing quality issues by designing processes and then enforcing them 'downwards' onto the manufacturing line, into the warehouse, and so on.
QC is product-oriented, at the cutting edge, and focused on identifying quality issues in manufactured products that could affect customer satisfaction.
Another way to understand this quality assurance vs quality control distinction is actions vs. results.
QA involves the actions which create the product, while QC is focused on checking the end result is satisfactory.
Several examples of each type of activity are detailed below.
QA processes:
- Documentation
- Audits
- Supplier management
- Personnel training
- Change control
- Investigation procedures
QC procedures:
- Batch inspection
- Product sampling
- Validation testing
- Laboratory testing
- Software testing
3. System (QA) vs. parts (QC)
Quality assurance control systems are the topline methods and procedures which are used to safeguard quality standards.
Quality control systems measure the efficacy of the underlying parts, including the outputs of the system.
QC efforts may be focused on parts used to create a final product, such as raw materials from a supplier.
The QA system for quality management, being more proactive as we've seen, may dictate various activities to make sure those inputs are consistently safe and effective at source, such as auditing suppliers and batch sampling raw materials.
4. Creation (QA) vs. verification (QC)
Another way to understand quality control vs assurance is the kind of activity involved in each discipline.
The result of QA activities is a roadmap for creating high-quality products. It involves defining standards for product design, manufacture, packaging, distribution, marketing, and sales.
QC involves the verification of products post-manufacture and before distribution, confirming their safety and efficacy.
5. Entire team (QA) vs. dedicated personnel (QC)
Quality assurance activities involve the entire business. Every member of a life sciences organization is responsible for QA activities by following SOPs and policies. While the quality management system (QMS) is generally the responsibility of the quality unit and the leadership team, QA activities involve standards for training, documentation, and review across the workforce.
QC, as a subset of QA, is generally the responsibility of certain dedicated personnel within the organization whose duties include following SOPs for things like product verification, inspection and testing.
QC staff should follow SOPs for quality control, then document their findings based on standardized procedures for product testing and process validation. These findings then flow back to the original processes, where QA activity can be performed to sharpen and improve them.
FURTHER READING: Quality management vs quality control vs quality assurance
QA vs QC: the role of an eQMS for quality assurance and quality control
Neither QA or QC are optional, and it’s impossible to say which of QA vs QC is more valuable. QA involves creating standards and processes to create a safe, effective process. QC activities validate the product itself.
As you've probably guessed by now, maximizing the effectiveness of your quality management system depends on a healthy mixture of quality assurance vs quality control activity.
Too little QC?
Your company gets stuck with pie-in-the-sky policies and procedures which bear no resemblance to the actual output of what your company is providing to customers. Defects and weaknesses are missed, patients are injured or even killed, customers are unhappy and leave, auditors have pages of nonconformances to note down, and your company is at the risk of fines, recalls and shutdowns.
Too little QA?
Your product quality might be steady and controlled, but what about checking, expanding, refining and sharpening your processes to make the wider business stronger? Without a mechanism for proactively getting under the hood of your processes with audits, upskilling your employees with training, or building fresh process documents, your company gets overly static and doesn't hit its full potential.
Unfortunately, modern quality professionals in regulated industries like life science are often struggling to get the quality assurance vs quality control balance right.
Our 2024 global survey of thousands of life science quality professionals found over half of respondents could allocate no more than 25% of their working time to QA tasks.
60% did no QC activity at all.

At the same time, manual admin continues to take up inordinate amounts of time in organizations reliant on legacy quality management tools like paper and spreadsheets.
Companies are forced to choose between QA vs QC.
And in turn, quality and continuous improvement initiatives suffer, holding companies back from their full potential.
Perhaps this is part of the reason why almost 30% of our respondents plan to ‘definitely’ adopt an eQMS in the next 12 months. Electronic quality management systems digitize and automate your quality system, slicing non-value-add and admin time from your working week and giving you the headspace to dedicate to both quality control and quality assurance - and the operational benefits that come with both in harmony.
QC and QA are better together, and they’re best when both are incorporated into a single, best-in-class electronic quality management system (eQMS) that connects your business.
Cloud-based QMS software for life science companies ensures your business can both assure and control quality, with document, training, design control, quality event and supplier management functionality.
Qualio's QMS is built specifically for start-up and scale-up life science companies. If your company needs a scalable, user-friendly eQMS platform to make the QC vs QA headache go away, just get in touch with us.