What is ISO 15189? A complete overview
If you operate a medical laboratory, you'll encounter the requirements of ISO 15189 sooner or later.
ISO 15189 is a crucial international standard for modern medical lab operation, and an important milestone in your quality and compliance journey.
So what is ISO 15189? We'll explore the standard's requirements, and how you can get accredited, in detail here.
Table of Contents
- Introduction to ISO 15189 standard
- Scope of ISO 15189
- Benefits of ISO 15189 accreditation
- ISO 15189 accreditation process
- ISO 15189 certification
- Finding ISO 15189 accredited laboratories
- Tips for successful ISO 15189 implementation
- Challenges in implementing ISO 15189
- The journey towards accreditation and continuous improvement
Introduction to ISO 15189 standard
Let's start at the very beginning: with a topline flyover view of ISO 15189 and what the standard actually entails.
What is ISO 15189?
ISO 15189 is the international standard that outlines the quality management system and competence requirements that apply to medical laboratories.
The most recent update to the standard was made in 2022 to align more closely with ISO 17025:2017, the standard for the competence of testing and calibration laboratories.
Medical laboratories who are accredited to ISO 15189:2012 have a period of three years (until December 2025) to transition to ISO 15189:2022, while labs looking to be newly accredited to ISO 15189 will need to comply with the 2022 version.
Scope of ISO 15189
Who is ISO 15189 certification intended for?
Let's examine the scope and focus of the standard to find out.
Application to medical laboratories
According to the ISO 15189 standard itself, medical laboratories are labs that examine:
“…materials derived from the human body for the purpose of providing information for the diagnosis, monitoring, management, prevention and treatment of disease, or assessment of health.”
These materials may include (but are not limited to) microbiological, immunological, biochemical, immunohaematological, haematological, biophysical, cytological, tissue and cells, and genetic material.
Examples of the types of labs that would pursue ISO 15189 certification are hospital laboratories, laboratories offering blood transfusion services, or contract labs used to carry out medical testing.
ISO 15189 also applies to point-of-care testing (POCT) providers.
POCT is the testing and analysis of patient specimens outside of a clinical lab, near or at the site of patient care. It's usually performed by clinical staff, such as nurses and doctors, but may also be performed by the patient themselves.
Medical devices like a blood glucose monitor or pregnancy test would be classed as a POCT device, and the provider would fall within the scope of ISO 15189.
The POCT requirements previously contained in ISO 22870 have now been incorporated into the revised ISO 15189, with ISO 22870 withdrawn.
Exclusions & limitations
ISO 15189 is a general standard applicable to all medical laboratories, regardless of their speciality, focus area or geographical location.
While it provides a solid foundation for quality management in a medical laboratory, some specialised laboratories may require additional accreditation or certification specific to their field to address unique technical or regulatory requirements.
One example of an additional regulatory requirement would be CLIA (Clinical Laboratory Improvement Amendment) certification issued by the FDA for medical laboratories.
It's also important to note that getting ISO 15189 accredited isn't mandatory in territories like the US, but it is in about 60 other countries worldwide.
Depending on where you're based, then, getting ISO 15189 certification is either highly recommended or flat-out unavoidable.
Benefits of ISO 15189 accreditation
If it isn't mandatory, why should you bother getting ISO 15189 accredited?
There are a few good reasons.
Quality assurance and patient safety
Because ISO 15189 performs an important quality assurance function that maximizes the integrity of your operation, getting ISO 15189 certification lets your customers know that the results your lab generates for them are accurate, reliable and trustworthy.
This, in turn, provides assurances to health care providers and patients that any treatment decisions based on the results you provide are sound.
Healthcare providers (HCPs) can use results from ISO 15189 accredited labs in their decision-making process to identify risk factors and predispositions to diseases, confirm or reject a diagnosis, guide the treatment management and monitor the effectiveness of a patient’s treatment.
Being a trusted laboratory operation therefore has an important knock-on effect on patient wellbeing and safety.
International recognition
ISO 15189 is a globally recognised accreditation. Often, companies who outsource medical laboratory testing will use ISO 15189 accreditation as a minimum requirement before a lab can be added to their Approved Supplier List.
For example the UK’s NHS requires that labs providing screening services for certain diseases MUST have ISO 15189 certification. And, as touched on above, 60 countries have made ISO 15189 part of their mandatory medical laboratory accreditation requirements, with that number growing steadily.
Continuous improvement
The benefit of ISO 15189 is that it requires the laboratory following the ISO 15189 standard to continuously improve its management system.
The opportunities for improvement can be identified in a number of ways which are required for as part of the ISO 15189 standard:
- Internal audit findings (Clause 8.3.3)
- Complaints (Clause 7.7)
- Corrective actions (Clause 8.7)
- Management reviews (Clause 8.9)
- Suggestions or feedback from employees, patients and users (Clause 8.6.2)
- Analysis of data (data inputs outlined various clauses)
- Risk assessments (Clause 8.5)
- Review and use of policies and procedures (various clauses)
- Review of overall objectives (Clause 5.5)
- External evaluation and assessment reports (Clause 7.3.7.3)
Senior laboratory management is responsible for ensuring that the laboratory participates in continuous improvement activities. The laboratory management is also required by the ISO 15189 standard to communicate to employees the lab’s improvement plans and goals.
Once the laboratory identifies areas for improvement, the lab should prioritize improvements based on the risk posed to the lab results, as well as patient and employee safety.
Once implemented, you should then evaluate the effectiveness of the improvement actions and ensure they've had the intended effect.
Cost savings & efficiency
Poor quality management systems in a medical laboratory will lead to higher costs and less efficiency.
One example of this is the use of resources to repeat testing when errors occur. By identifying errors through the non-conformance process required by ISO 15189, labs can put in place corrective and preventive actions and evaluate their effectiveness so that those errors do not occur again.
Failure to evaluate the effectiveness of the corrective actions in accordance with ISO 15189 can lead the lab to perpetuate a costly cycle of poor quality and inefficiency.
If a lab does not have a robust non-conformance and CAPA program, not only will it waste resources (both time and money) on repeat testing, but more errors mean more investigations, more root cause analysis and more corrective actions to be put in place, costing the medical lab even more in the long run.
More errors and delayed results hurt the medical lab’s reputation, will lead to more complaints (again, more investigations, more CAPA!) and most importantly, may put the lives of people at risk based on the potential for misdiagnosis and/or inappropriate treatment plans.
ISO 15189 accreditation process
Now we've explored the benefits of getting ISO 15189 accredited, here's how to actually complete the process and secure ISO 15189 certification.
Pre-assessment
Once an initial application has been made to a conformity assessment body (CAB), your medical laboratory may have to undergo a pre-assessment. The pre-assessment may be recommended so that a CAB can determine how ready your operation is for ISO 15189 accreditation.
During a pre-assessment visit, the CAB will perform a review of your QMS documentation to identify any significant gaps or misunderstanding of the requirements of the ISO 15189 standard.
Assessment
Once you've cleared the pre-assessment process, it's time for the assessment process proper to begin.
There are a few stages to consider here.
Document review
This initial assessment is usually broken down into two stages which occur on separate days. The document review may be done prior to the Stage 1 audit, or during it.
As part of the document review, the assessor will request your quality management system (QMS) documentation to check if it meets the requirement of ISO 15189.
If any deficiencies are found, the assessor will include these on your Stage 1 audit report.
Onsite assessment
Now for the scary bit! Your assessor will complete two onsite assessments of your lab operation: Stage 1 and Stage 2.
The Stage 1 audit, sometimes referred to as a 'desktop audit', is used to determine how your medical laboratory has implemented and documented each element of ISO 15189 compliance.
The Stage 2 audit is a more detailed dive into the evidence of your QMS to ensure the procedures around various elements of the ISO 15189 standard are implemented.
Stage 1
During the Stage 1 onsite assessment, the assessor will evaluate the readiness of your medical laboratory for accreditation, and assess your current QMS and documentation if not done so before the Stage 1 audit.
The assessor will use the information gathered during the Stage 1 assessment to determine if you're ready to progress to the Stage 2 assessment.
After completion of the Stage 1 audit, the assessor will document any findings of a discrepancy between the requirements of the standard and your identified processes.
The assessor will communicate those findings to the medical laboratory and will ensure that the Stage 2 audit date allows for enough time for the medical laboratory to resolve any areas of concern identified.
Stage 2
The Stage 2 audit will verify the compliance of your medical laboratory to all requirements specified in the standard.
During the Stage 2 audit, the assessor will look at how a process works, where it happens and who performs the tasks, then explore the evidence that it has occurred.
They'll also interview your personnel in the lab who complete the tasks, and your senior management - who, remember, are responsible for the overall QMS.
Once the Stage 2 audit has taken place, the assessor will discuss any findings at the close-out meeting and issue a report outlining the findings.
Your medical laboratory will then have a set amount of time to document the findings as non-conformities and provide a report back to the assessor with evidence that the findings have been addressed or that corrective actions have been put in place to address the findings.
The assessor will usually indicate the format in which they want the corrective action report structured.
Decision on accreditation
Now you've passed through the assessment, it's time for your auditor to make their final decision.
Once they've received the corrective action report back from you, they'll make a decision on the awarding of ISO 15189 accreditation for your medical laboratory.
The outcome of the decision may be one of the following:
- Awarding of accreditation
- Awarding of accreditation subject to the corrective actions being implemented adequately
- A need to re-audit or perform a follow-up visit at a later date to witness and verify that the corrective actions are in place
- No awarding of accreditation, meaning that the medical laboratory requires a complete re-audit
If accreditation is awarded, your medical laboratory will be notified in writing with a certificate of accreditation which includes the scope of the accreditation.
Well done!
Surveillance and reassessment
Once your medical laboratory receives ISO 15189 certification, you'll need to undergo surveillance audits (usually every 6-12 months, depending on your certification body) and then a full re-assessment or re-certification audit (anywhere from 2-4 years, depending on your certification body).
The surveillance audit focuses on assessing your laboratory’s ongoing compliance with the requirements of ISO 15189. Think of it as a 'check-up' and a way for your certification body to keep an eye on you.
This may include the review of internal audits, management reviews, continuous improvement actions and review of changes, and ensuring operational control is maintained.
Your assessor will also check to make sure any actions arising out of previous audit findings have been adequately completed.
The reassessment audit, on the other hand, is a more comprehensive process.
It involves a more thorough examination of your medical laboratory’s entire quality management system, processes, documentation, facilities, the technical competence of the laboratory, and overall compliance with ISO 15189.
When your medical laboratory completes a successful reassessment audit, you'll be issued a new ISO 15189 certificate.
ISO 15189 certification
And there you have it. Successful completion of your document review, both stages of your onsite assessment, then your surveillance and recertification audits, is your key to securing and maintaining ISO 15189 certification.
To go deeper into the quality management requirements for modern medical laboratories, read our guidance blog post:
FURTHER READING: Mastering quality management in laboratory environments (12 essential techniques)
Finding ISO 15189 accredited laboratories
Perhaps you're searching for a reputable medical laboratory to outsource some of your operation to.
As we've seen, ISO 15189 is an internationally recognised stamp of quality and integrity, and so finding an ISO 15189 accredited lab is a good way to source a potential business partner.
ISO 15189 accredited laboratories list
Unfortunately, there's not really a single list of all 15189 accredited laboratories to search.
Some government agencies may have a central list depending on your location, but most of the time you'll need to find an accreditation body’s website (BSI, TÜV SÜD, and so on) then search their database of accredited labs.
So if you want to check up on a particular laboratory's accreditation status, you’d have to know what accreditation body they used in the first place.
How to check if a laboratory is ISO 15189 accredited
Most medical laboratories who are ISO 15189 accredited will sing it from the rooftops (and rightly so!) Often, they'll have the ISO 15189 accredited logo or a copy of the ISO 15189 certificate available to download on their website.
If you want to confirm their certificate is still valid, the most reliable source is the website of their accreditation body, as touched on above.
If you're unable to find a list of accredited medical laboratories on their website, you can reach out to the accreditation body directly for confirmation of a valid ISO 15189 certificate.
Tips for successful ISO 15189 accreditation
Thinking of pursuing ISO 15189 certification yourself?
Here are some top tips and key ingredients to get in place to make your accreditation journey as fast, smooth and successful as possible.
Leadership commitment
A culture of quality starts from the top.
Not only does ISO 15189 directly call out the responsibilities of the laboratory director in terms of implementation of the quality management system, implementing any change requires the management to communicate why the lab is implementing ISO 15189, what ISO 15189 is, and what the benefits are to ensure employee buy-in.
The leadership team should also ensure that they regularly communicate progress and updates on the status of the ISO 15189 implementation.
This can be done through team meetings, emails, newsletters or other internal communication platforms. The leadership should also highlight employees whose involvement and work has had a positive impact on the implementation of ISO 15189 in the laboratory as this maintains motivation and engagement.
Download our culture of quality toolkit for some helpful resources
Employee involvement & training
Engaging employees in the implementation of ISO 15189 is crucial for the successful adoption and integration into your medical laboratory’s operations.
Employees, especially SMEs in a particular area, should be involved in discussions around the implementation of ISO 15189-required processes and should be encouraged to share their insights and contribute ideas that will improve these processes.
In some cases, cross-functional teams will be required to develop processes that impact different areas. This encourages teamwork and promotes the idea that quality management is everyone’s responsibility.
To further engagement in the implementation process, employees should be offered training and education programs so that they better understand the ISO 15189 requirements, and their roles and responsibilities in the implementation process.
This may include training on quality management principles, documentation procedures and other technical training relevant to their role.
This not only leads to better implementation but is also a requirement of ISO 15189: employees need to be aware of the quality management system and understand their contribution to its effectiveness, the benefits of continuous improvement in the laboratory, and the consequences of non-conformity.
Adaptation to the laboratory's specific needs
Cut-and-paste compliance isn't helpful.
To successfully implement ISO 15189 in your medical laboratory, it's important that your specific scope of services, patient population and other regulatory requirements are fully understood.
Prior to implementation, it's beneficial to perform a gap analysis to identify any areas where your laboratory’s current practices and processes deviate from the requirements of ISO 15189.
This allows your leadership team to prioritize and address gaps most relevant and critical to the laboratory’s operations and quality management system. These gaps should be prioritized based on the impact on patient safety, compliance with your relevant regulations and overall laboratory performance.
Seeking support and guidance
If you need more support and guidance on getting ISO 15189 accredited, your accreditation body can provide information on their specific accreditation process.
Some companies with limited experience in ISO 15189 often choose to employ a consultant who specializes in ISO 15189 to help them get up and running with compliance to the standard.
And if you're thinking of implementing an electronic quality management system (eQMS) to accelerate and simplify your ISO 15189 preparations, consider Qualio's pre-built life science eQMS content pathways.
Qualio specialists can load your shiny new eQMS with pre-configured ISO 15189 templates and documentation for faster and smoother compliance. Our friendly specialists can also support you with targeted strategy sessions for advice on implementing ISO 15189 in your medical laboratory.
Challenges in implementing ISO 15189
It's important to consider potential ISO 15189 certification challenges early, so you can proactively plan how to tackle them.
Here are a few to think about.
Costs and resource implications
The cost and resource implications of implementing ISO 15189 accreditation can vary based on the size and complexity of your laboratory, the current quality management system and the level of operational maturity already in place.
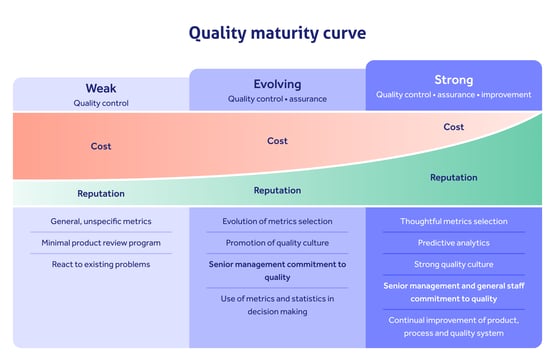
In terms of personnel, ISO 15189 emphasizes the competence of laboratory staff. Meeting these requirements may involve recruiting additional personnel, providing further training and conducting regular competency assessments. These activities will all impact on staffing costs, training expenses, and time needed to perform training and assessments.
Learn how to make life science quality training easy, natural and automatic
Further employee resources may need to be diverted from regular tasks to implement the processes required of ISO 15189. This includes the creation and maintenance of laboratory documentation such as quality manuals, procedures and records.
Finally, personnel resources will be required to perform the quality control and quality assurance practices dictated by ISO 15189. This includes audit management, equipment maintenance and calibration, and activities associated with continuous improvement such as monitoring, analysis of data and the implementation of corrective actions.
The accreditation process itself will have a financial cost for your medical laboratory as well. Medical laboratories may incur costs associated with assessment fees, travel and accommodation for assessors and the costs associated with implementing corrective actions identified during the assessment process.
Other financial considerations may include the need for new equipment or systems, and/or updating of facilities.
Cultural & organizational changes
As stated before, any change must be led from the top down and this is no different for ISO 15189 certification.
To create a culture of quality, it’s essential that your leadership team champions the implementation, provides the necessary resources and support and involves the laboratory employees in the process to foster a sense of ownership and engagement.
When this happens, it allows quality to become ingrained in the mindset and practices of all laboratory employees.
Organizationally, ISO 15189 emphasizes a process approach to medical laboratory operations. This means that the laboratory must shift from a task oriented mindset to a holistic view of processes, focusing on efficiency and effectiveness.
Communication and collaboration will become key as personnel from different teams or areas need to work together to implement ISO 15189.
Your leadership team must be focussed on breaking down organizational siloes and establishing channels and processes for the exchange of information, feedback and ideas.
Encouraging and nurturing collaboration and teamwork not only enhances problem-solving, but also fosters a learning culture and improves overall laboratory performance.
Timeframe for implementation
It's also important to understand how long your ISO 15189 accreditation journey will take.
Generally speaking, it usually takes between three and six months to achieve ISO 15189 certification once the assessment process is started with your accreditation body.
There are a few factors that may impact on the speed in which your medical laboratory can implement ISO 15189. These include:
- The size and complexity of your laboratory
- Resources and personnel availability
- Competing priorities
- The maturity of your QMS
- The level of compliance already in place
- The number of issues discovered during your pre-assessment or Stage 1/2 audits
- Employee training and competence
It is essential that any medical laboratory looking to obtain ISO 15189 certification develops a realistic implementation plan based on these factors.
A well-structured plan with clear milestones, resource allocation and regular monitoring can help manage the timeline effectively and ensure successful ISO 15189 implementation within your desired timeframe.
The journey to accreditation and continuous improvement
While the journey to become ISO 15189 accredited may appear daunting at first, any laboratory that secures ISO 15189 certification won't regret it.
ISO 15189 certification enhances your laboratory's credibility, reputation and international recognition, providing a competitive advantage and attracting fresh patients, healthcare providers and research collaborations.
And it promotes a culture of continuous improvement, ensuring that your laboratory operates at an optimal level, delivering reliable and accurate test results while prioritizing patient safety and quality.
To make your ISO 15189 certification journey even easier, consider how to optimize your toolset and operational processes. ISO compliance software like Qualio brings your key ISO 15189 accreditation ingredients - documents, training, CAPAs, suppliers and analytics - into a single cloud-based system that connects your entire organization.
Book a demo of our ISO 15189 eQMS software to see how it works.
