A comprehensive review of the best 21 CFR Part 11 software in 2024
There are multiple 21 CFR Part 11 software providers that can help you meet the requirements of the FDA's electronic record regulations. But a baseline of compliance isn’t necessarily maximizing the of value your 21 CFR Part 11 software. To move to the top of your market and position your company for future growth, a thorough audit of your available options is crucial.
It's important to look beyond what software will help you reach compliance alone and instead, determine what tool will help you build a modern, quality-centric approach to your quality processes.
FDA guidance on software for electronic records and signatures is somewhat broad. This gives you the opportunity to adopt a system that meets your 21 CFR Part 11 software requirements and unlocks additional benefits, too.
For instance, 21 CFR Part 11 compliance software might also offer sharper document collaboration, smarter document control, better data security, or (ideally) all three.
While the best 21 CFR Part 11 software for your company depends on your specific business make-up and requirements, there are a few characteristics common among all of the leading options for 21 CFR Part 11 compliant software.
Let's take a look at the features and qualities you should be looking for - then dive into the market leaders offering those features.
Table of Contents
What makes 21 CFR Part 11 software compliant?
21 CFR Part 11-compliant software is any document management software that meets the U.S. FDA's requirements for the acceptance of electronic records and electronic signatures.
These standards aim to ensure that electronic records and signatures used in your business are just as trustworthy and reliable as paper equivalents.
Current good manufacturing practice (cGMP) does not require the use of software for the submission of documents or signatures. The FDA provides only generalized guidelines about the types of technologies which can meet these requirements.
However, quality management software (eQMS) that offers document management capabilities is the most common approach to meet compliance requirements for 21 CFR Part 11 software.
This software is adopted by FDA-regulated organizations such as medical device, pharma, biotech, and contract research or manufacturing organizations who must comply with 21 CFR Part 11.
5 benefits of the best 21 CFR Part 11-compliant software
As you hunt for the best 21 CFR Part 11 software for your business, it's important to understand the key benefits and advantages you want to unlock.
What can you expect from a reputable, functional 21 CFR Part 11 system?
Let's take a look.
1. Automatic compliance
Managing electronic records without an integrated software system can be a nightmare.
From Excel spreadsheet version control to capturing legally binding signatures, there's no way to maintain Part 11 compliance without constant, time-consuming manual upkeep.
In a fast-moving and constantly evolving life science organization, documents and records made in one corner of the business can be out-of-date in another as soon as they're produced - and it falls to the poor quality or compliance manager to sift, sort and organize to get a semblance of order and compliance in place.
Your 21 CFR Part 11 software system, then, should include automated guardrails and 'checks' on document activity to make compliance natural and automatic.
This should include, for instance, locking down document versions to prevent multiple simultaneously circulating versions, or mandating e-signature capture as record updates are made.
The more features like this, the less you need to think about Part 11 compliance.
2. Single source of truth
Your Part 11 software should offer as close to a single source of truth as possible.
It's no good having version control if you need to bounce across multiple digital repositories to source your key quality documents.
You may be, strictly speaking, compliant, but you're lacking the full easy traceability and control that an FDA inspector will expect to see - and it makes your day-to-day more difficult.
Beware 21 CFR Part 11 systems which offer full compliance within the system, but which then force you to take your documents out of the software for key activities like editing and collaboration.
Prioritize a system which offers a protective, compliant framework across 100% of the document lifecycle.
3. Integration with broader QMS
Electronic records and signatures are not a goal in themselves.
In a robust, functional quality management system, your document stack is the connective tissue of data holding your entire QMS together and propping up your other quality management activities.
You could buy a 21 CFR Part 11-compliant software system offering great record and document control.
But if that system offers only record management, you're then relying on a host of other systems for the rest of your QMS.
Not only does this approach multiply costs, it forces you and your teams to circulate across different platforms and systems for their daily work, introducing silos and potential information gaps.
The best 21 CFR Part 11 software won't only be document management software. Look for a holistic system offering other functionality like training, supplier or quality event management.
4. Auditability
Let's face it: you want 21 CFR Part 11 software because you know, sooner or later, an FDA inspector will walk through your doors and start checking your electronic record and signature processes.
That inspector will want at-a-glance visibility of your processes and controls, so they can be confident you know what you're doing.
The best CFR Part 11 software should have that magic 'auditability factor', combining a clear UX with logical structuring to make it instantly clear how your records and signatures are handled.
Your system should offer easy access to a third party like an auditor to go in and check themselves, as well as having the ability to be easily 'walked through' and narrated by your designated contact throughout the inspection.
5. Return on investment
Like any software, your CFR Part 11 system investment should work hard for your company and offer some financial payback.
As we've just explored above, it can be worth buying a more holistic software platform offering other QMS functionality alongside Part 11 compliance to consolidate your costs.
Functional Part 11 software will also offer clear and measurable return on investment by replacing time-heavy paper and spreadsheet work with automated, digitized record and signature processes.
AIIM estimates that in companies with suboptimal information management, employees waste half an hour every day just looking for the records and data they need to do their jobs.
That's the equivalent of 1 in 16 of your staff doing no work at all!
There's also another, hidden benefit to Part 11 software.
Since controlled and compliant records should underpin everything you do as a regulated company, they naturally reduce the risk of other business problems like defects and rework if they're properly maintained in a Part 11-compliant system.
That means a lower cost of poor quality and reduced risk of recalls, downtime and regulatory fines.
Recommended 21 CFR part 11 software requirements
If you're asking, "What's the best 21 CFR Part 11 compliant software?" you're not necessarily asking the right question.
There are no software products designed exclusively for 21 CFR Part 11 compliance. Most commonly, FDA-regulated organizations achieve compliant signatures and records through QMS software.
Since the FDA guidance on meeting Part 11 requirements does not outline specific functionality of software, organizations should consider evaluation criteria like usability and product agility.
The best 21 CFR Part 11 software offers features for FDA-compliant electronic signatures and records, as well as features that enhance quality management and collaboration capabilities.
Add the following functionality to your 21 CFR Part 11 software requirements list:
1. Easy validation
Computerized system validation, now known as computerized system assurance, is required for organizations regulated by the FDA or European Medicines Agency.
Effectively, organizations must validate their computer systems, such as software used for quality management, to prove it complies with 21 CFR Part 11 and other regulations such as :
- 21 CFR 210-211
- 21 CFR 820
- 21 CFR 600
- 21 CFR 1271
Beyond requiring validation and providing some guidelines, the FDA doesn't provide specific instructions on how to approach 21 CFR Part 11 validation.
But they do recommend the 'least burdensome approach'.
Although organizations used to rely on documents like Installation Qualification (IQ), Operation Qualification (OQ), and Performance Qualification (PQ), these no longer reflect the iterative nature of modern software validation, and the FDA encourages businesses to adopt a risk-based, critical thinking approach and work with their software providers to make validation as straightforward and logical as possible.
Nevertheless, with the wrong vendor, validation can quickly become extremely complicated, especially with on-premise software systems or software which has been heavily customized to meet an organization's requirements.
If your 21 CFR 11 software contains a considerable amount of custom codes or DIY integrations with other systems, you may face operating issues or huge complexities each time you have to update the software or patch a security issue. Your vendor may offer limited support for validation, or charge a costly consulting fee for this process.
Before investing in software for compliance with 21 CFR Part 11 and other FDA requirements, investigate the vendor’s approach to software validation at the time of installation, operation, and performance, as well as built-in mechanisms for revalidation as part of the change control process.
Ideally, cloud software vendors should offer simple revalidation packages as a client service.
Your software vendor should also be prepared to keep up with industry best practice and regulatory expectations, such as those laid out in the Second Edition of GAMP 5 released in mid-2022, its supporting 'Enabling Innovation' Good Practice Guide, and the FDA's stance on validation and quality management.
2. Robust document control features
There's no such thing as software which can guarantee or 'certify' compliance with FDA 21 CFR Part 11.
Organizations bear the responsibility of Part 11 compliance, including procedural or administrative controls such as software.
However, software which provides the necessary support for 21 CFR 11-compliant electronic records and signatures is likely to have robust document control features, including revisions tracking and audit trails. The best software includes both technical and collaboration features to meet requirements and help your organization work more effectively.
3. Technical features
Your 21 CFR Part 11 software should be able to completely manage your organization's electronic records, including document revisions and approvals with trustworthy date and time stamps.
Records should be archived per your company's policy instead of removed or deleted from the system.
The software should provide a comprehensive audit trail of all document actions, including how users or groups of users have interacted with documents, and document workflows.
Finally, per FDA requirements for electronic signatures, the software should require users to enter their credentials for documents which require a legally binding signature to ensure the integrity of a user's signature.
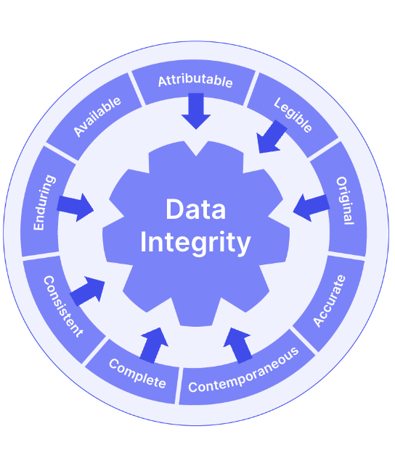
All these features contribute to a robust ALCOA+ document approach which helps your business embed Good Documentation Practice (GDocP) as well as simply complying with Part 11.
4. Collaboration
The best compliant document control software helps organizations work more effectively by improving team communication on FDA-required documents.
This should include automated notifications and reminders for document contributors, and the ability to leave in-line comments during document revisions. Cloud-based software enables your team to collaborate globally.
The best document control features meet FDA requirements for electronic records and signatures, but they shouldn't fit the bill in a way which 'feels' compliant or adds unnecessary complexity to your organization's workflow.
Ideally, it should make global collaboration around trustworthy, compliant electronic documents a more streamlined and useful part of your process.
5. Authenticated electronic records
FDA guidance for the authentication of electronic records and signatures requires that software "must employ at least two distinct identification components such as an identification code and password," according to Subpart C of Section 11.200.
Effectively, your organization needs to be prepared to prove a user's identity based on a signature if the trustworthiness and authenticity of your records or signatures are ever brought into question.
Simply providing a form for your users to type their name and the date isn't enough. Multi-factor authentication is an essential software feature for FDA 21 CFR Part 11 compliance and the security of your sensitive data.
There are several possible approaches to user authentication, which can be loosely categorized as the following:
- Type 1 - "Something You Know" - Passwords, PINs, or secret questions
- Type 2 - “Something You Have” - Texting a Code to a Mobile Phone
- Type 3 - “Something You Are” - Biometric validation of fingerprints or retinas
Software should require, at a minimum, that users enter their password or a PIN before creating an electronic signature.
Depending on your organization's requirements, you may choose to increase security by moving to a multi-factor authentication model which includes a mobile phone text or validates the user's device.
Ask a prospective software company how they verify a user's identity to ensure trustworthy electronic signatures, and how this information is reflected in the audit trail.
6. Strong password requirements
21 CFR Part 11 requires that organizations establish 'access control' to closed systems and create an audit trail, but provide little guidance on specifics.
Each user account which has access to the system must be associated with a unique username and password combination, and per FDA guidance, organizations should maintain access control by creating username and password combinations which limit a user's data access and capabilities based on their role.
Currently, there are no recommendations on adopting software which requires strong passwords or periodic password changes.
Just meeting the FDA's non-aggressive guidance for passwords is likely to present a liability to your organization's data security.
In fact, 80% of information security incidents with data loss involved weak or stolen passwords, according to the Verizon DBIR. Weak passwords can present an enormous risk that compromise your organization's data security.
Lazy passwords can be easily guessed or shared by colleagues, which may not have malicious intent. In some situations, coworkers may use a colleague's account since it's "easier" than asking for different permissions or a password change. However, these innocent workarounds compromise the authenticity of records and signatures and the trustworthiness of your audit trails.
Your software should include features to enforce effective password policy, including:
- Unique passwords
- Password encryption
- Enforced password selection
- Password expiration
- Security questions
Users should be required to choose a new password every 30-90 days, which consists of a unique combination of letters, numbers, and special characters. The software should also lock user accounts when an incorrect password is entered repeatedly.
Ensure prospective vendors don't offer weak security policies around user passwords, such as a policy of emailing lost passwords directly to a user instead of enforcing a password change.
7. Simplicity
Compliance with 21 CFR Part 11 and other FDA cGMP can be complicated.
However, your software shouldn't feel add unnecessary burden or effort to your company's quality management processes.
It also shouldn't feel like a compliance software or a product which adds laborious steps to your workflows. It should make compliance fade into the background of a user-friendly product for collaboration and natural, automatic quality management.
A simple product should help your organization exceed requirements and adapt with agility to new cGMP requirements.
The definition of software which offers 'simplicity' can vary significantly depending on an organization. A product which is the right type of "simple" for a start-up could be far too lightweight for an enterprise with a vast catalog of approved products for market.
However, some general signs that a product is 'simple' are:
- Clean, flat UX
- Broad functionality set that makes one product suitable for multiple uses i.e. document, training, event management
- Linked processes for end-to-end process visibility
- Customized workflows and features to streamline quality management processes
- Built-in validation and revalidation packages
- Simple, closed-loop reporting for continuous improvement
- An intuitive, user-friendly software experience on desktop and mobile
If your organization is evaluating software for compliance with FDA 21 CFR Part 11, consider the time-to-value as a component of simplicity.
How easy is the software to activate? How much engineering and configuration is required ahead of time?
Ideally, the platform should require minimal customization, aside from configurations to meet your organization’s requirements. To achieve compliance and other possible benefits quickly, fast-track cloud software vendors that offer a quick implementation and value out-of-the-box.
8. Scalability
The ability to scale software to company growth and new FDA requirements is a critical feature, especially at fast-growing scale-ups and start-ups in FDA-regulated industries. If your organization is in the pre-market phase of researching and developing new products, your system will need to scale to new capabilities, such as new:
- Users
- Work sites
- Products
- Clients
- Suppliers
- Processes
- Workflows
Also, to provide the capacity to scale, a software vendor should make it affordable for clients to grow. A final component of scalability is the capability of the software to integrate with existing systems or easily transfer to a new system.
The top 5 21 CFR Part 11 software systems in 2024
Several vendors offer software with each of these must-have features outlined above.
Remember: the best software for your organization is the one which helps you maintain continuous compliance with FDA cGMP and matches your other requirements for budget, ease of use, functionality and speed of implementation.
1. MasterControl
MasterControl is a total quality management suite with broad adoption among enterprise customers, including several major regulatory agencies.
This solution is focused on helping large organizations manage large global portfolios of products, bring new products to market at greater speed, and increase organizational efficiency.
User reviews report satisfaction with linked quality processes and extensive document control capabilities.
This software is likely best suited for substantial enterprises, due to user-reported extensive requirements for configuration, a reported high cost, and a steep learning curve which may require vendor-supported training.
2. TrackWise
This software is positioned as an 'out-of-the-box' solution for compliance, which is built on the Salesforce platform.
Users can gain access to prebuilt workflows, validated product releases, and built-in compliance with 21 CFR Part 11.
Client reviews on G2 Crowd report satisfaction with the product’s prebuilt offerings for workflows and the software’s ability to efficiently scale existing workflows and documents to new processes.
Users report dissatisfaction with the vendor’s post-sale service and customer support.
3. Qualio
Qualio is the first cloud-powered eQMS designed to embed natural, automatic compliance with FDA 21 CFR Part 11, Part 820, ISO 13485, GxP and more.
Over 600 start-up and scale-up life science organizations in 80 countries use Qualio to not only comply with 21 CFR Part 11, but to build a robust document management system within a broader digital quality framework.
Qualio provides the full gamut of world class document management features, from audit trails and e-signatures to templates, traceability and cloud-based access.
Qualio's secret weapon, though, is its fully native in-app document editor.
Where other eQMS platforms force users to check quality documents in and out of their systems to edit them, adding a layer of risk to your 21 CFR Part 11 electronic record compliance, Qualio's complete document editor offers the full range of collaboration and document-building functionality needed to keep you entirely in one system alone.
Qualio becomes a single source of truth offering a closed one-stop shop for your entire document lifecycle, with your quality documents guardrailed from drafting to archiving for airtight CFR Part 11 compliance.
User reviews report superior client support from the vendor, a comprehensive feature set and a natural, intuitive user experience which maximizes adoption.
4. Intellect
Intellect makes the most of its configurability credentials, positioning itself as one of the most flexible eQMS platforms on the market.
It also offers an EHS solution and a no-code 'Platform' product for building your own business applications.
Its document control functionality offers the core ingredients for 21 CFR Part 11 compliance, but the lack of a native editor means documents must be downloaded, edited in a third-party system like Word, and then re-uploaded into the eQMS.
This 'check-in, check-out' process could introduce potential integrity weaknesses if followed incorrectly.
Customer reviews mention high pricing - Intellect is an enterprise product unsuitable for smaller companies - as well as slow, cumbersome support, unhelpful user guides and inconsistent helpfulness.
5. Veeva
Veeva offers a complex suite of quality, regulatory, CRM, safety, clinical and patient management systems.
Its Vault eQMS product contains a 'QualityDocs' section offering a cloud-based document library for CFR Part 11 compliance.
The sheer scope of Veeva's product suite and its layers of functionality mean your CFR Part 11-compliant document system could be easily connected to other areas of your Veeva system, such as a fully-fledged LIMS.
But some customers report difficulty formatting and naming documents, with different naming conventions required inside and outside Veeva Vault. These difficulties seem to increase with larger documents, and could introduce some potential weaknesses into your electronic record integrity and compliance.
Choosing 21 CFR Part 11 compliant software
When choosing 21 CFR Part 11 compliant software, balance your requirements with features like easy validation and robust document control before you make a final decision.
And ensure a vendor is the right size for your company's current size, budget, and growth goals to avoid an overly costly investment, complex implementation or a product which can't scale.
Schedule a demo with us to ask questions, dive into our product offering and ensure you've selected the best 21 CFR Part 11 software for your needs!
