Understanding the major pharmaceutical regulations you need to know
Pharmaceuticals offer a critical, often life-saving capability to patients across the globe. For that reason, pharmaceutical regulations are an essential core of the industry.
From maximizing patient safety to assuring drug quality and manufacturing integrity, pharmaceutical industry regulations structure and standardize expectations of how modern pharmaceutical businesses should operate.
It's essential that your business understands, digests and applies the regulations in the pharmaceutical industry that apply to you.
Here's a comprehensive list of the key pharmaceutical regulations you need to know.
International regulations and guidelines
There's a complex patchwork of pharmaceutical regulations to understand and get to grips with. Some are mandatory territory-specific requirements, others are voluntary models of best practice to strengthen your operation.
Let's start by exploring the voluntary standards laid down by the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, or ICH.
Because they're international and go beyond the requirements of other, mandatory quality benchmarks, ICH pharmaceutical regulations are the best way to build a standardized and widely applicable framework of up-to-date best quality practice.
ICH Guidelines (International Council for Harmonisation)
The most commonly adopted ICH standards form the so-called 'tripartite model' of ICH Q8, Q9 and Q10.
The ICH commonly refers to all three standards together, and simultaneous compliance with all three is recommended.
Why? The pharmaceutical regulations of ICH Q8, Q9 and Q10 form a mutually interacting and supporting trifecta of pharmaceutical quality best practice.
ICH Q8 maps out the international best practice for pharmaceutical development and so-called 'quality by design', or QbD.
Its current iteration, revision 2 or 'R2', focuses primarily on how drugs are manufactured. In short, by focusing on the quality target product profile of your drug and the critical quality attributes that need to be controlled to get you there, you can form a standardized, repeatable and risk-controlled manufacturing methodology that churns out safe, identical, pure and efficacious pharmaceutical product.
ICH Q9, on the other hand, is all about managing risk across your pharmaceutical operation.
Lots of regulations in the pharmaceutical industry include a risk focus - after all, uncontrolled drugs and undetected compliance issues can have a deadly effect.
But ICH Q9 is your best pick of the pharmaceutical regulations if you want an airtight and focused quality risk management strategy.
ICH Q9 lays down a systematic approach of risk assessment, control, communication and review, focusing on integration of both quality and risk management.
ICH Q10, meanwhile, can be used to knit the ingredients of ICH Q8 and Q9 together into an overarching pharmaceutical quality system, or PQS.
ICH Q10 combines GMP requirements with the ISO 9001 structure (more on both of those below!) of continuous improvement underpinned by management engagement.
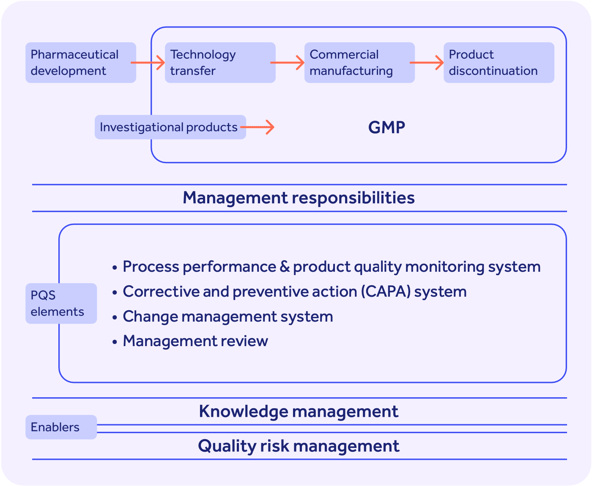
In a robust ICH Q10 system, all 4 stages of your drug's lifecycle, from development to discontinuation, are covered by a risk- and knowledge-based quality approach.
Process performance and product quality are continuously monitored, with CAPAs, management reviews and change management all deployed in tandem to spot problems, fix them and continually improve.
ICH Q8, Q9 and Q10 are the most commonly applied and well-known of the ICH pharmaceutical industry regulations.
But like ISO standards, there are dozens of more niche ICH pharmaceutical regulations you could apply to your business.
As well as other 'Q' quality standards like Q2 (analytical variation) and Q12 (lifecycle management), there are also 12 'S' safety guidelines, 21 'E' efficacy guidelines and 15 'M' multidisciplinary ICH benchmarks to consider, from S4 (toxicity testing) to E2 (pharmacovigilance) and M6 (gene therapy).
The bundle of ICH pharmaceutical regulations applicable to you depends heavily on the precise context and nature of your operation - so do your research!
Good Clinical Practice (GCP)
ICH E6 (R3) is a useful crossover point between the ICH section and the next: good clinical practice.
Good clinical practice (GCP) is part of the broader quality umbrella of GxP pharmaceutical regulations. It's a key benchmark encapsulated in ICH E6, and then borrowed into multiple pharmaceutical regulations across the planet. For this reason, it shouldn't be seen as 'just' an ICH guideline - GCP is now a mandatory part of how modern clinical trials are operated.
It should be seen as a kind of agreed-upon ethical and quality standard which has translated into legislation like the US HHS 45 CFR Part 46, the UK's Medicines for Human Use (Clinical Trials) Regulations 2004 and the European Union's 2005 Directive on Good Clinical Practice.
It has, of course, also found its way into FDA pharmaceutical regulations, with FDA 21 CFR Parts 50, 54, 56, 312 and 314 all mapping out GCP requirements in the US.
Good clinical practice guidelines aim to guarantee that clinical trials are performed in a high-integrity, ethical way based on the World Medical Association's Declaration of Helsinki.
Subject rights, wellbeing and above all safety should be front and center as the primary consideration for all clinical actors. Trials themselves should be built on a risk-benefit focus, without bias or manipulation of results.
GCP should set the quality benchmark for any pharmaceutical product's clinical trials, offering a shared pharmaceutical regulation framework for sponsors, institutional review boards, researchers and regulators to follow.
Good Manufacturing Practice (GMP)
Good manufacturing practice sits at the center of pharmaceutical quality assurance, and is the next of the GxP regulations in the pharmaceutical industry that you should know about.
It's often shortened to GMP, while in the United States it's known as cGMP or 'current' good manufacturing practice.
The American cGMP label captures nicely the evolving and shifting nature of good manufacturing practice expectations.
In the US, FDA 21 CFR Parts 210, 211 and 314 are the relevant cGMP FDA pharmaceutical regulations.
In the EU, EudraLex Volume 4 comes into play. The WHO's TRS 986 Annex 2, meanwhile, is a useful international pharmaceutical guideline to brush up on.
In short, good manufacturing practice pharmaceutical regulations are designed to ensure that drugs are made in a way that guarantees their purity, safety, integrity and efficacy.
As such, they span the entire scope of your manufacturing operation - from the personnel performing your manufacturing tasks to the equipment and facilities you utilize.
RELATED READING: What is cGMP in the pharma industry?
Good Laboratory Practice (GLP)
Any pharmaceutical operation involving a laboratory will need to consider and apply the next benchmark in our list of pharmaceutical industry regulations: good laboratory practice, or GLP.
The FDA's 21 CFR Part 58, the EPA's 40 CFR Part 160/792, the OECD's GLP principles and the WHO's GLP handbook are all useful starting points for your GLP research.
GLP pharmaceutical regulations mandate a clean and safe laboratory underpinned by standardized ways of working and high levels of internal technical expertise. As a result, your outputted products, results and conclusions should be of maximum trustworthiness and integrity.
PIC/S
Our next set of international pharmaceutical regulations is the so-called PIC/S guidelines.
The Pharmaceutical Inspection Co-operation Scheme (PIC/S)'s pharmaceutical regulations are a set of international standards and cooperation mechanisms designed to ensure that pharmaceutical products are manufactured with quality and safety at the forefront. GMP and the manufacturing line are the primary focus of PIC/S.
Remember the WHO GMP standards we touched on above?
PIC/S GMP pharmaceutical regulations are broadly similar, but apply in participating PIC/S countries such as the US, Canada, Australia, Argentina, Brazil, Japan, the UK, and those in the EU.
If you aren't in these areas, WHO GMP guidelines apply.
PIC/S pharmaceutical regulations are generally more modern and more rigorous than their WHO counterparts. They include a greater focus on key areas like quality risk management, hygiene, sanitation and product recalls.
ISO standards
Now, let's move onto ISO regulations in the pharmaceutical industry.
ISO standards are the most common, most well-known and most broadly applied quality management standards because of their internationally applicable nature.
There are thousands of ISO standards, some pertaining to incredibly niche business contexts and operational formats.
For this reason, there are a few key ISO pharmaceutical industry regulations worth knowing and exploring.
Let's start with the broadest and most basic of all!
ISO 9001
ISO 9001 sets the foundation for quality management systems in any type of business.
For this reason, it's as good a starting point as any for pharmaceutical companies early on in their quality management journeys.
ISO 9001 promotes standardized processes, risk management and continuous improvement, enhancing efficiency and product quality by applying a standardized quality management system.
This standard is your gateway to ensuring safe and effective medicines while maximizing customer satisfaction.
But it's also worth noting that ISO 9001 is a basic, industry-agnostic framework. Compliance to ISO 9001 alone won't be sufficient for any pharmaceutical business looking to hit the stringent quality and compliance objectives of the industry.
Think of ISO 9001 as the building blocks or baseline upon which to 'layer' compliance with more specific pharmaceutical industry regulations.
ISO 17025
ISO 17025 is a great example of a more niche, industry-focused ISO pharmaceutical regulation.
It specifically targets testing and calibration laboratories, which can be critical in pharmaceutical quality control and research.
ISO 17025 compliance ensures accurate, reliable results, crucial for regulatory compliance and product safety.
Pharmaceutical labs adhering to ISO 17025 can confidently validate their product quality claims, ensuring precision and consistency with a standardized laboratory QMS.
FURTHER READING: How to get an ISO 17025 certification
ISO 15378
ISO 15378 focuses on pharmaceutical packaging materials, making it a useful pharmaceutical regulation to strengthen your Good Manufacturing Practice or Good Distribution Practice adherence.
Compliance with ISO 15378 guarantees the quality and safety of your packaging materials, in turn reducing contamination risk, product degradation and adulteration concerns.
Pharmaceutical companies can rely on robust ISO 15378 compliance to safeguard product integrity from manufacturing line to patient use.
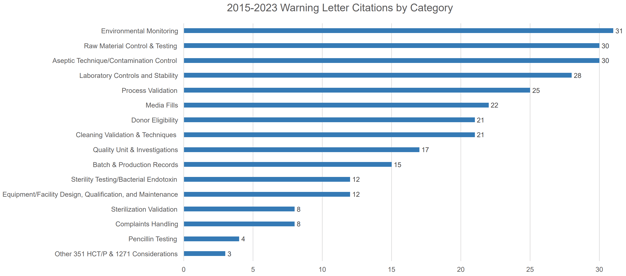
As environmental monitoring, control of raw materials and general contamination controls continue to top the causes of FDA pharmaceutical warning letters, having strong packaging controls in place is a good way to insulate your business from the risks of a common quality slip-up.
ISO 31000
Next on our list of pharmaceutical regulations is ISO 31000.
ISO 31000, like ISO 9001, is a fairly broad and non-industry-specific standard.
But because it provides a comprehensive best practice framework for risk management, it's useful for helping pharmaceutical companies identify and mitigate potential hazards connected to their operations.
ISO 31000 adherence can therefore be instrumental in maintaining drug quality by offering a model for managing the risks associated with development, production and distribution.
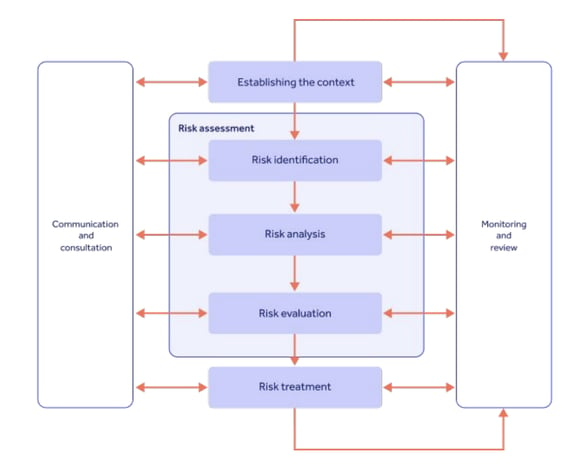
TOP TIP: Pair ISO 31000 with ICH Q9 compliance for a world-class pharmaceutical risk management system!
ISO 14644
ISO 14644-1 and ISO 14644-2 set cleanliness standards for cleanrooms and controlled environments in pharmaceutical facilities.
Compliance with these pharmaceutical industry regulations is vital to prevent contamination, ensuring that your drug products are manufactured in environments meeting stringent cleanliness criteria.
ISO IDMP
This bundle of ISO pharmaceutical regulations was simmering in the background for about 5 years before being launched in 2017.
ISO's IDMP (Identification of Medicinal Products) initiative aims to ensure that pharmaceutical products are defined and categorized in a standardized way, so that medicinal information can be more easily exchanged between global regulatory and healthcare bodies.
Supply chains and drug shortages are a major area of focus across the planet for pharmaceutical industry actors. The FDA's Quality Management Maturity program and the EU's new Pharmaceutical Legislation both have shortage-busting as a key objective.
The logic of the ISO IDMP pharmaceutical regulations, in a similar way, is to help global drug supplies be even more readily identified and distributed, strengthening international supply chains, sharpening pharmacovigilance and clinical trial operations, and insulating from the risk of shortages.
The IDMP pharmaceutical regulations comprise:
- ISO 11238: substance identification
- ISO 11239: dosage form & route of administration
- ISO 11240: units of measurement
- ISO 11615: medicinal product identifier
- ISO 11616: pharmaceutical product identifier
These 5 standards cover the 4 key domains of so-called 'SPOR' master data in pharmaceutical regulations: substance, product, organization and referential.
The EMA has already made compliance mandatory in the EU.
The US FDA has some parallels, too: the U.S. National Drug Code offers similar outputs to ISO 11615, their Unique Ingredient Identifier is similar to ISO 11238, and the Unified Code for Units of Measure conforms to ISO 11240's requirements.
In short: both of the major pharmaceutical markets of the US and EU have the spirit of these ISO pharmaceutical regulations codified into law. Understanding and applying their requirements is therefore crucial if you plan to operate in these territories.
Ensuring drug safety and efficacy
What's the common thread running through our list of pharmaceutical regulations?
All put reliable, repeatable drug safety and efficacy front and center of your operation.
The exact make-up of pharmaceutical industry regulations applicable to you depends on your business context and location, but adherence to your pharmaceutical regulations in all cases requires a robust and effective quality management system.
From managing documents and employee training to handling quality events like deviations and OOS investigations, a strong pharmaceutical QMS is your ticket to continuous improvement and full operational compliance with your pharmaceutical industry regulations.
As you research the pharmaceutical regulations you need to meet, start by assembling the operational ingredients of a QMS (or, better yet, an eQMS) to launch your business towards natural, automatic and long-term compliance.
