Guide to GAMP 5, data integrity and quality by design GxP manufacturing PDF guide
Understand what modern GxP manufacturing looks like - and how to optimize it at your business.
Get the guide now. No email required!
Download our GAMP 5, data integrity and GxP manufacturing PDF guide to:
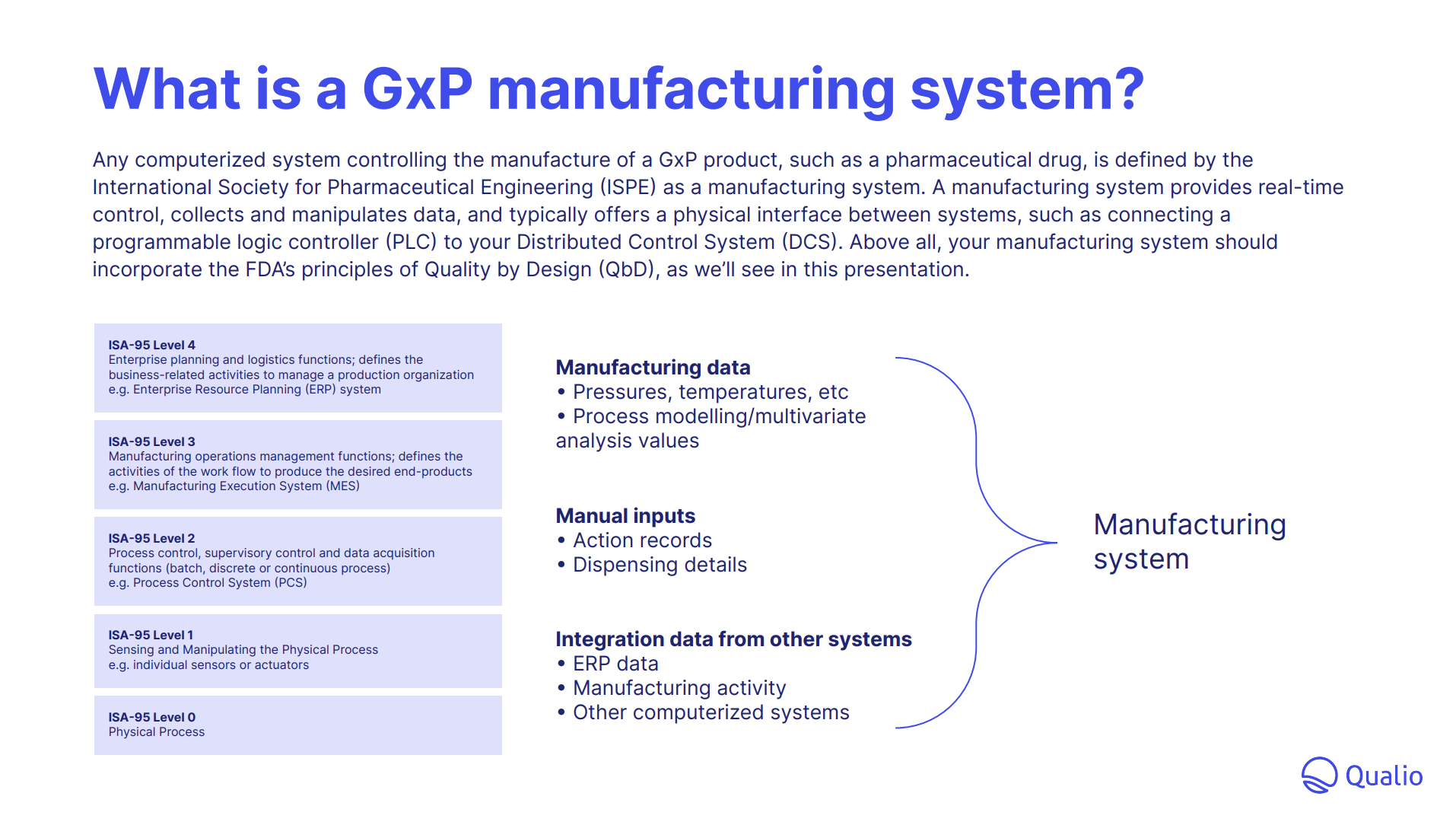
- Understand the key ingredients of the modern GxP manufacturing system
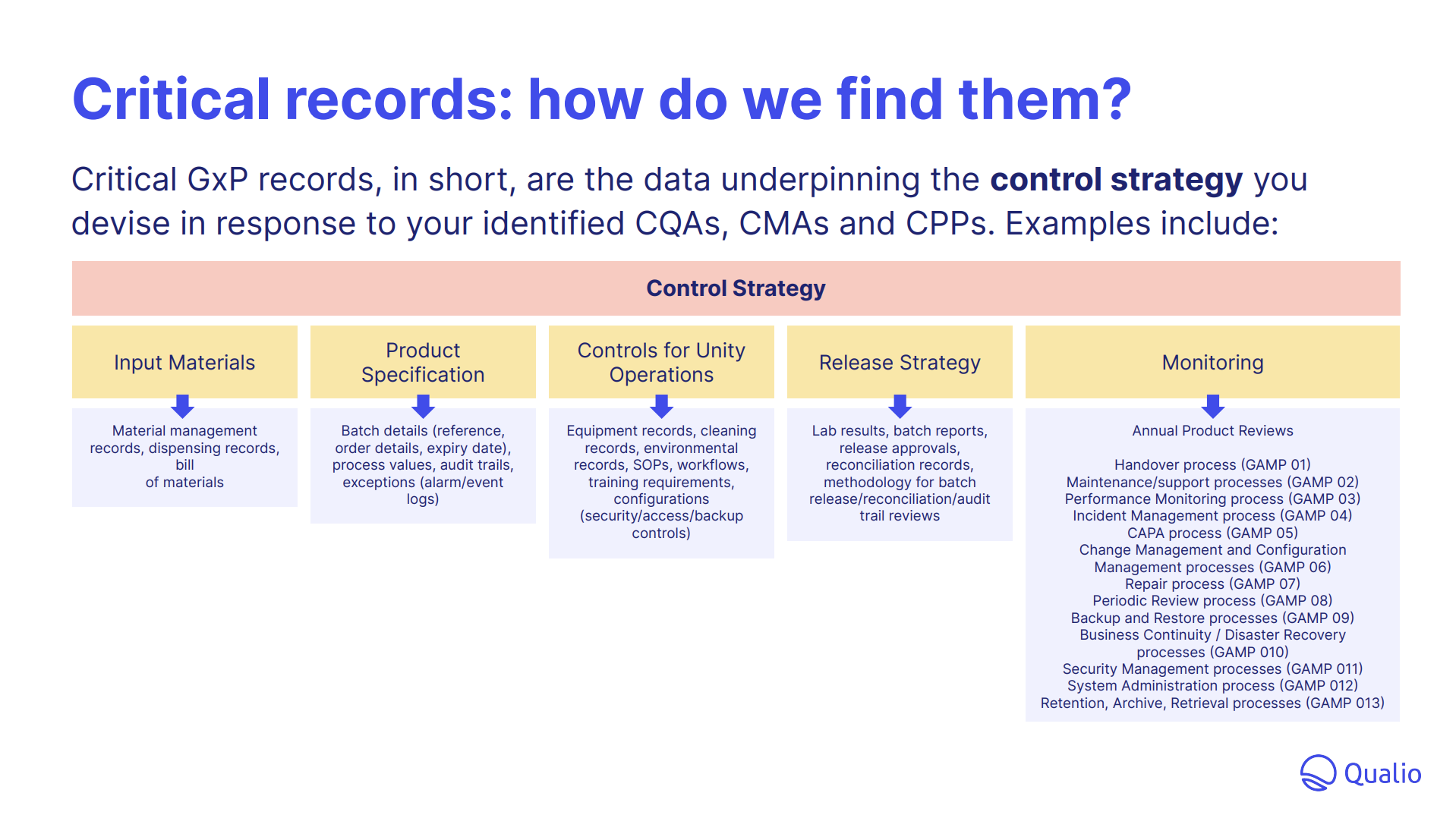
- Embed critical quality touchstones, from QTPP to CQAs, into your operation
- Learn how to confidently manage manufacturing risk and apply appropriate tools for best-in-class pharma, biotech and medical device manufacture
What you'll get:
GxP blueprint
Understand what modern GxP and GAMP mean for your quality and compliance obligations
Confident manufacturing
Master the quality of your product, and apply the right tools for continuous quality improvement
Roadmap to QbD
Understand what quality by design (Qbd) entails, and how to ensure it sticks around in all layers of your business