Tips for passing your MHRA inspection
If you plan to market a medicinal or medical device product in the United Kingdom, you'll need to ensure your Medicines & Healthcare Regulatory Agency (MHRA) inspector leaves your business happy.
Like any regulatory inspection or audit, MHRA inspections can be stressful, time-consuming black marks on the calendar if you don't prepare properly.
Molly and Lola from the Qualio quality team sat down with Karen Hue, Head of Quality & GxP Compliance at Oxford-based 30 Technology, to discuss best practice tips for passing your MHRA inspection with flying colors - and enjoying yourself in the process!
Let's dive in.
Prepare the 4 pillars
Lola recommends paying attention to 4 key preparation areas before any announced or unannounced inspection:
1. Internal training
Prepare the company and your colleagues for what's coming.
Assemble a Standard Operating Procedure (SOP) for both announced and unannounced inspections for teams to access and follow on the big day - and ensure staff are trained and retrained on them.
It's also important to ensure everyone is familiar with the terminology and phraseology you can expect to hear from your MHRA inspector.
And crucially, key members of the company should be allotted an appropriate role to follow as the inspection kicks off:
- The authorized escort is the constant point of contact who escorts the inspector around your site
- The scribe takes notes and records the inspection sessions
- The runner finds and brings relevant documents and colleagues to the inspection room
- The subject matter expert (SME) takes inspector questions about their particular area of expertise
Top tip
Train your colleagues on appropriate levels of conversation to keep the inspection focused.
Small talk isn't necessary, nor is detailed explanation of documents or going beyond the questions asked by your inspector.
Let your objective evidence do the talking!
2. Pre-inspection requests
Your MHRA inspector will request specific documents before your inspection begins.
Determine what format they're happy to receive these documents in. Assemble a file of digital or paper documents as requested, or, if you have an eQMS, prepare a document within the system, link to other relevant documents, and grant access.
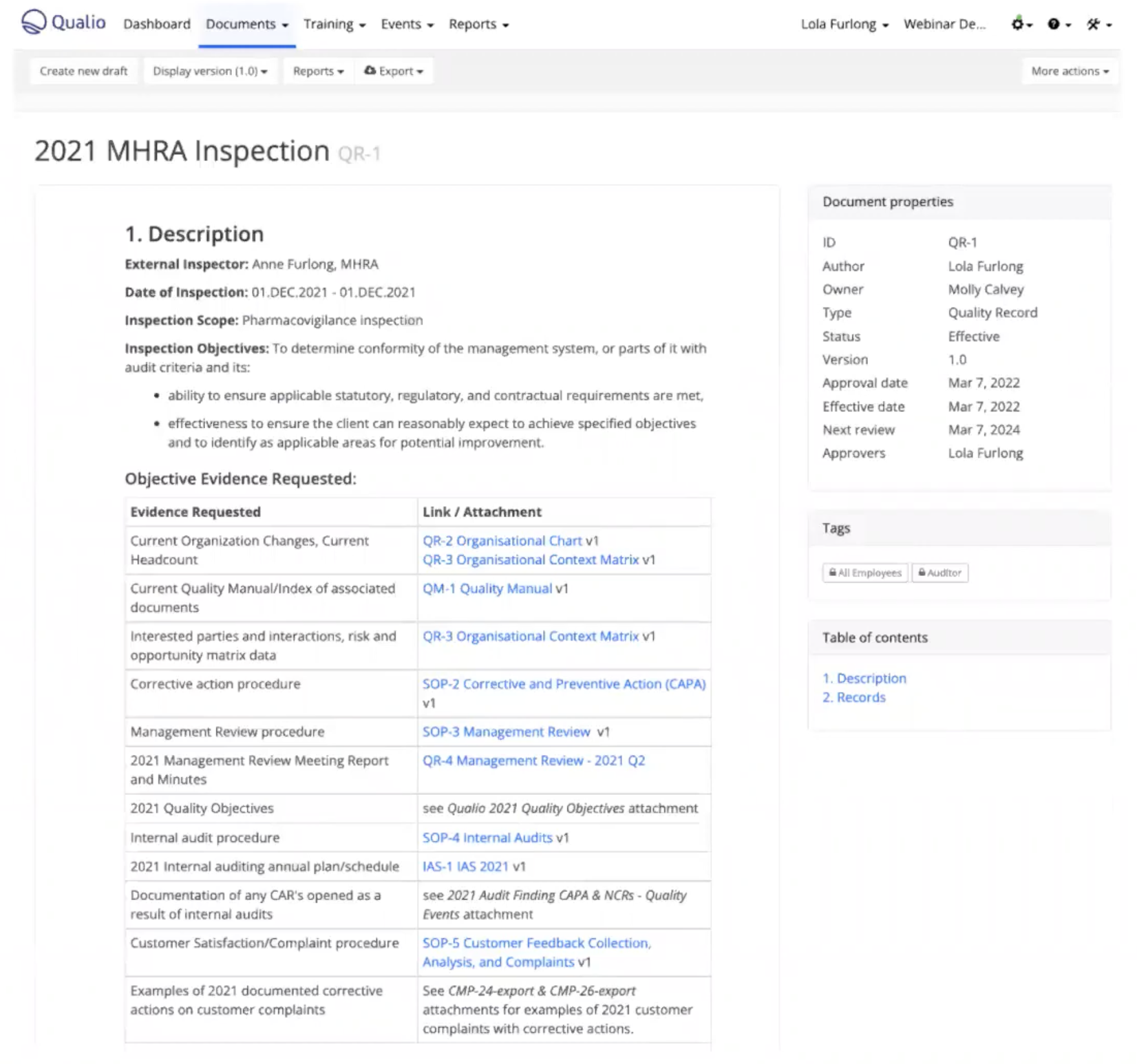
Example of a pre-inspection document prepared in Qualio, with links to key information like organizational charts, CAPA SOPs, the quality manual and more
3. Back-up plan
Imagine welcoming your MHRA inspector through the door just as your Internet connection goes down.
However unlikely, this nightmare should be prepared for by downloading key digital documents ahead of time. That way, nothing can disrupt the flow of your inspection or leave you high and dry with nothing to show.
4. eQMS prep
If you're a Qualio user, simply build an 'Inspector' or 'Auditor' tag and apply it to any document you'd like your inspector to see when they access your eQMS.
Unfiltered access to your entire document stack and to live system tasks and notifications could make it more difficult for the inspector to home in on the handful of key documents they might wish to access - so set viewing permissions accordingly.
And it's also worth considering preparing a brief training guide to help your inspector log in and use your eQMS if they aren't familiar with it.
Expecting a remote audit or inspection? Read our tips!
Always be inspection-ready
It's all very well having a preparation dialogue with an inspector for an announced and scheduled inspection.
But being constantly ready in the event of an unannounced inspection is vital too.
Karen Hue of 30 Technology uses Qualio to guarantee constant inspection-readiness by maintaining a core of objective evidence documentation - SOPs, manuals, quality objectives and so on - which are always tagged appropriately.
A surprise inspector can simply provide their email address as they walk through the door and be granted appropriate and targeted access in seconds.
Karen also recommends being considerate of any inspector's potential computer literacy, by investing in an eQMS that's intuitive and easy to use and providing a 'crib sheet' of training resources to get them familiar and comfortable quickly.
Applying a set timeframe to your inspection SOP can also make sure you drive a smooth, controlled introduction process in the early stages of your inspection:
"We have a rule that within 30 minutes of an inspector ringing our doorbell, they should be sitting in our boardroom reviewing the first set of documents."
Karen Hue, Head of Quality & GxP Compliance, 30 Technology
Be agile for responding to findings
Did your inspector find something? Don't panic!
Your inspector's job is to help your company be better, not to block your route to market.
Treat your findings as positive improvement opportunities, not as a slap on the wrist.
And above all, get to work to address any inspection findings as quickly as you can with a robust and controlled follow-up process.
For Karen and the 30 Technology team, there's no point waiting for the inspector's report if something objectively fixable was flagged mid-inspection: give yourself a mechanism for your scribe to record, categorize and combine action points, and assign them to relevant personnel, on the day of the inspection.
Post-inspection CAPA sharing can be a balancing act between demonstrating continuous improvement and raising your bar unnecessarily high, so act accordingly:
"What we're careful not to do is 'over-give' continuous improvement information.
We make sure we answer the inspector's findings and provide the appropriate CAPA - and anything else we feel we need to do is logged and acted on without overburdening ourselves in terms of what the inspector will expect to see when they come back."
Karen Hue, Head of Quality & GxP Compliance, 30 Technology
Ensure you review the MHRA's guidance for responding to a post-inspection letter, and if you're using an eQMS like Qualio set system workflows for responding to findings. This offers a string of key benefits:
- Enforceable due dates and timelines ensure you can demonstrate what your inspector needs to see in good time
- Personnel and team assignment allows you to close out findings in the most efficient and sensible way
- System action reviewer(s) can make sure findings have been properly closed out before you notify the MHRA
- An audit-trailed and fully traceable set of CAPA actions will build in real time as you work through your findings, allowing you to prove at your next inspection how you've progressed from the previous
"Using an eQMS - using Qualio - has been a great asset for us."
Karen Hue, Head of Quality & GxP Compliance, 30 Technology
More learning
Want a deeper dive into MHRA inspection best practice?
Watch the webinar recording with Molly, Lola and Karen on demand now.
Just starting or early on in your quality journey? Get helpful tips and fascinating insights from experts straight to your inbox.
