The ultimate ISO 9001 overview: quality management systems
Are you preparing for ISO 9001 certification? Wondering what the standard means for your organization?
As of 2025, over 2 million global organizations are certified as compliant with the requirements of ISO 9001:2015.
The ISO 9001:2015 standard for quality management systems is the fundamental, baseline set of requirements for modern quality management.
Comply with ISO 9001, and your organization is perfectly positioned for any niche, supplementary industry quality requirements.
Here's everything you should know about ISO 9001.
Introduction to ISO 9001
If you want to establish a holistic and functional quality management system from scratch, ISO 9001:2015 is the perfect place to begin.
ISO 9001 lays out the core fundamentals of the modern quality management system (QMS) and the key ingredients for providing products and services that consistently meet the requirements of your customers.
By itself, ISO 9001 compliance won’t provide the niche life science quality management requirements needed for a medical device, pharmaceutical or therapeutic business.
But it will give you the foundational groundwork upon which all your QMS activity is built.
Crack ISO 9001, and your business is empowered to move onto any other quality standard – and be recognized as an established, quality-conscious organization as you do so.
What is ISO 9001?
ISO 9001:2015 is an international standard, developed by the International Organization for Standardization (ISO), that maps out how to establish and run a quality management system.
In doing so, ISO 9001 provides a framework and a set of principles your organization can follow to ensure your products and services consistently meet customer requirements and enhance their satisfaction.
ISO 9001 meaning
ISO 9001:2015 follows a process-based approach to quality management, emphasizing the importance of understanding and meeting customer expectations, establishing clear quality objectives, then using them to continuously improve your processes and performance.
It sets out criteria for a quality management system that organizations can adopt regardless of their size, industry or location.
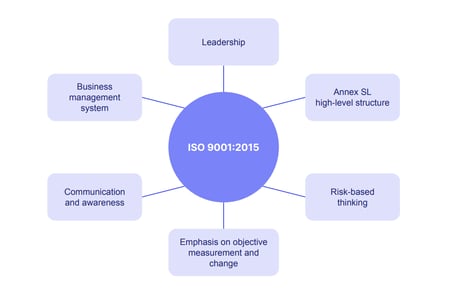
Key principles of the ISO 9001 standard
ISO 9001 embodies 4 key principles, as follows:
1: Adoption of a quality management system as a strategic organizational tool to:
- Consistently provide products and services that meet customer, statutory and regulatory requirements
- Demonstrate conformity to specified QMS requirements
- Address opportunities to enhance customer satisfaction
- Address both risks and opportunities associated with the context, objectives and strategic direction of your business
2: Embedding of quality management principles:
- Customer focus
- Leadership
- Engagement of people
- Process approach
- Continuous improvement
- Evidence-based decision making
- Risk-based thinking
3: Implementation of the process approach:
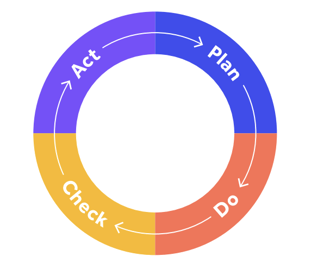
- Incorporating the Plan-Do-Check-Act cycle for repeatable interacting processes
4: Relationship with other management system standards:
- Annex SL: for consistency
- Aligns with other ISO standards for better integration
ISO 9001:2015: the latest revision
Why the '2015'?
The ISO 9000 body of quality management standards have gone through a series of revisions since their initial launch in 1987.
The 2nd, 3rd and 4th editions came in 1994, 2000 and 2008, before the current 5th edition rolled out in 2015.
What changed between the latest version and what preceded it? Let's take a look.
Changes in ISO 9001:2015
The so-called 'seven year-switch' saw a series of changes between ISO 9001:2008 and ISO 9001:2015.
Two extra clauses were added to bring the total to ten. (We'll explore those below.)
The product-based quality model of ISO 9001:2018 was upgraded to include business services, too.
A fresh focus on context was added, encouraging organizations to focus on the internal and external parties, factors and problems touching their quality systems.
New expectations around documented information emerged, with key QMS ingredients like objectives and scope added to the list.
Perhaps most significantly, a deeper focus on risk was enshrined. The basic risk expectations of ISO 9001:2018 were replaced with a model of more proactive, comprehensive pinpointing of both risk and opportunity, as well as documented steps to address and harness them.
Lastly, senior leadership are expected to play a more central role in ISO 9001:2015, with clear input, oversight and responsibility for the execution of the QMS.
Benefits of the 2015 revision
ISO 9001:2015 compliance demands more from pursuing businesses than the 2008 standard did, mapping out a more comprehensive, proactive model of modern quality management.
Leadership engagement, sharper risk and contextual focus, and an increased emphasis on both opportunity harnessing and continuous improvement turns your quality management system from a set of fairly static requirements into a more nimble, collaborative, robust and continually evolving platform for enhancing your customers' satisfaction.
Understanding the ISO 9001 requirements
Now that we've examined the general background and construction of ISO 9001:2015, it's time to unpick the standard's requirements in more detail.
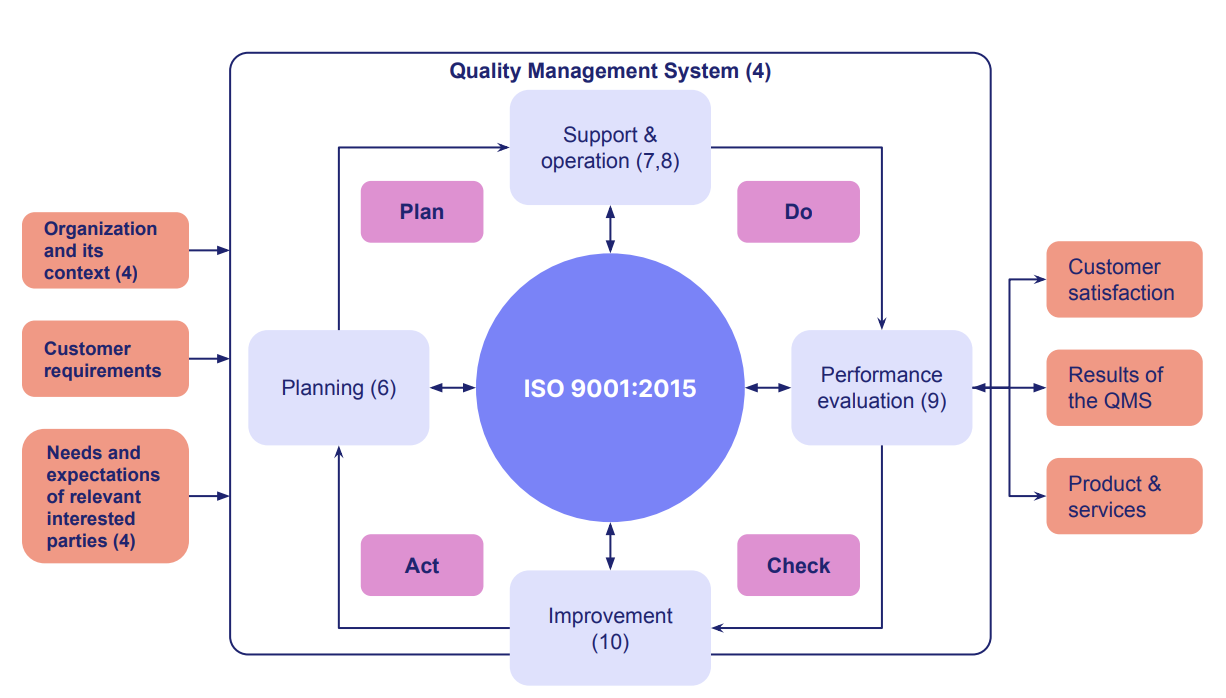
The ISO 9001 quality management system
Building a QMS that aligns with the ISO 9001 standard demands careful, synchronized execution of a number of key elements.
Quality management processes
“Consistent and predictable results are achieved more effectively and efficiently when activities are understood and managed as interrelated processes that function as a coherent system.”
— Introduction to ISO 9001:2015
Clause 4.4, ‘the QMS and its processes’, strongly suggests the adoption of a process approach for building and maintaining your ISO 9001 quality management activities.
The process approach focuses on how your QMS processes interlink and impact each other, and coordinating them into an ‘integrated and complete system’ underpinned by Plan Do Check Act.
The process approach, executed properly, helps your organization overcome a classic quality management problem which looks like this:
- Organizations are typically structured into departments, managed by a responsible department head
- Most departmental heads never interact with the customer, only internal stakeholders
- As such, they are divorced from how your customer base really feels
- Heads try to maximize the performance of their departments to the possible detriment of other departments
- Departmental KPIs compound the problem
In contrast, the process approach introduces 'horizontal' management, controlling processes which flow across departmental boundaries.
Someone is accountable for each process from start to finish.
They understand what the stakeholders in the process want, and have delegated authority to act to realize this.
Their first loyalty is to their assigned projects, products or services, not their own departments.
Read more about the process approach in ISO/TC 176, 'Guidance on the concept and use of the process approach for management systems'
Quality policy and objectives
A centralized, top-down quality policy, with measurable objectives, should structure and align your entire QMS.
Crucially, both policy and objectives should be aligned and compatible with the strategic direction and context of your organization.
And, as we've seen, customer centricity and maximizing customer satisfaction is crucial for ISO 9001 compliance.
Customer requirements should infuse into your policies, procedures and quality objectives:
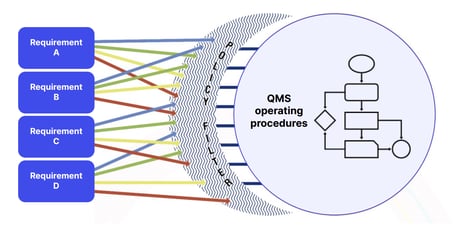
Management commitment
ISO 9001:2015, unlike ISO 9001:2008, places emphasis on the proactive, constant commitment of your management (or 'leadership') team to the execution of the QMS.
Your leadership team should:
- Inform everyone in your business of the importance and benefits of a certified QMS
- Tell everyone why they should participate in its effective implementation
- Ensure your quality policy and objectives are compatible with the strategic context of your operation
- Promote risk-based thinking in respect of the organization’s quality management system
- Make sure the management system achieves its intended outcome, and dedicate adequate resources accordingly
- Ensure the effectiveness of the quality management system
When your ISO 9001 auditor arrives, they'll expect to see evidence of managerial commitment to and responsibility for the quality agenda, including frequent management review sessions with documented outcomes.
FURTHER READING:
The 9 core elements of an ISO 9001 quality management system
Clauses in ISO 9001:2015
Now let's examine each of the ten clauses of the ISO 9001 standard to see what requirements they mandate.
1. Scope
The first three clauses of ISO 9001:2015 are scope, normative references and terms.
These are information clauses, rather than clauses that outline particular actions or major requirements. These clauses highlight the basic tenets of a functional quality management system.
The scope, the focus of Clause 1, sets out the intended outcomes of your management system. The outcomes are industry-specific and should be aligned with the context of your organization (which we'll see in Clause 4).
When considering your QMS' scope, remember that ISO 9001 compliance hinges on demonstrating the ability to consistently supply products and services that meet your customer, statutory and regulatory requirements.
“Output resulting from product realization” was removed from ISO 9001:2008 to reflect the new standard's changes to the definition of process and output.
2. Normative references
Provides details of the reference standards or publications relevant to the particular standard.
3. Terms & definitions
The terms and definitions section in Clause 3 outlines the rudimentary vocabulary and definitions from ISO 9001:2015.
Terms that you'll need to know to fully understand the basics behind 9001:2015 include ISO, standards, asset inventory, management, management system, policy, process approach, and quality management, among others.
ISO 9001:2015 defines the QMS as "an organization's system which implements policies and objectives into the processes that help improve a standard of quality."
The main changes from ISO 9001:2008 include:
- New focus on risk
- New mention of innovation
- Management responsibility › Leadership
- Purchasing and outsourcing › Externally provided processes, products and services
RELATED READING: The 7 fastest ways to fail an ISO audit
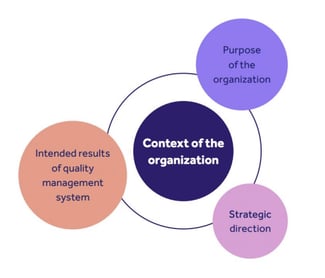
4. Context of the organization
Discusses the context of your QMS and how your business strategy supports this. Clause 4 determines why your organization is here.
It involves 4 key areas:
1. Understanding the organization and its context (new requirement!)
2. Understanding the needs and expectations of interested parties (new requirement!)
3. Determining the scope of the management system
4. Executing the management system
5. Leadership
Concerns the role of 'top management': the group of people who direct and control your organization at the highest level.
This clauses outlines the roles and responsibilities of leadership as it pertains to the adoption and implementation of your QMS.
The purpose of this clause's requirements is to unify your key stakeholders around key quality management objectives.
6. Planning
This phase addresses planning to address both risk and opportunity as it pertains to product development in conjunction with the quality management system.
This allows your organizations to address areas of high risk, and where applicable, activate risk prevention or mitigation activities.
By doing so, your organization can better identify areas of impact that might adversely affect your quality management system's ability to perform as expected.
Core components to consider include:
1. Actions to address risks and opportunities
2. Management system objectives and planning to achieve them
3. Planning of change
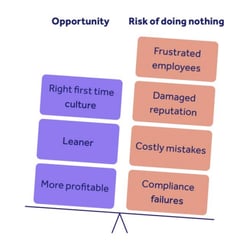
RELATED READING: Mastering risks and opportunities guide
7. Support
Focuses on getting the right resource to the right people, and getting the right infrastructure in place.
Consider your internal resources and competence, how awareness and communication will take place, and how documented information will be built, maintained and distributed across your organization.
8. Operation
The nitty-gritty: how to meet your customer requirements and execute your plans and processes. Clause 8 encourages you to consider risks associated with each product or service, as well as your customer requirements, feedback and any other statutory requirements.
Think about:
- Operational planning and control
- Specific requirements for each product and service you offer
- Design and development of products and services
- Control of externally provided processes, products and services
- Production and service provision
- Release of products and services
- How you'll control non-conforming output
9. Performance evaluation
Measure and evaluate your QMS to ensure that it is effective and to determine what, how and when things are to be monitored, measured, analyzed and evaluated.
Monitoring, measurement, analysis and evaluation, as well as internal auditing and management review, are all important ingredients to consider for compliance with Clause 9.
10. Improvement
By complying with Clause 9 and monitoring and measuring your processes, you're well set up to meet the continuous improvement expectations laid out in Clause 10.
Your organization should determine and identify opportunities for continual improvement of the QMS.
Non-conformities should be pinpointed and fixed with targeted corrective and preventive action (CAPA) to maximize customer satisfaction.
ISO 9001 implementation
How should you meet each clause's requirement?
Where do you even begin?
There are a few key steps to follow to implement a robust ISO 9001 quality management system in your organization.
Steps to implement ISO 9001
Here's a comprehensive flow process chart to help you tackle your ISO 9001 steps one-by-one:
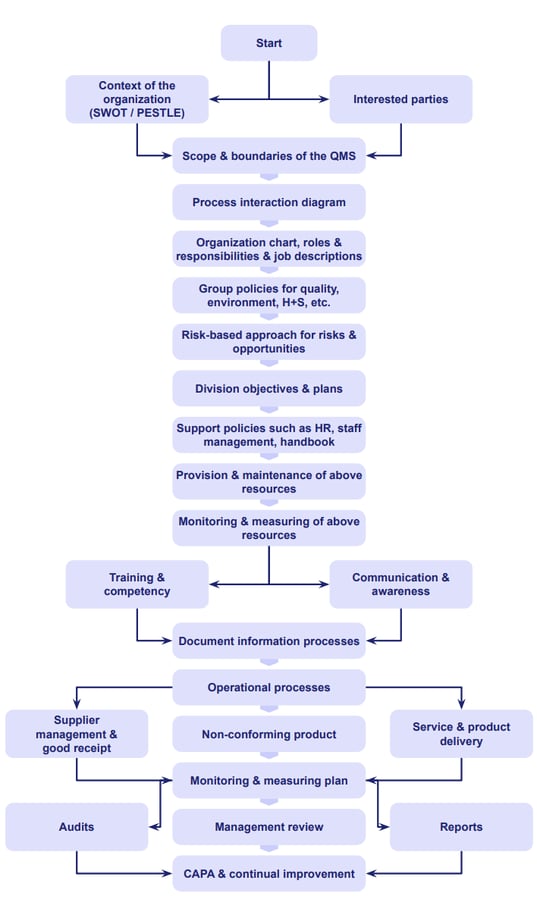
We've also assembled an ISO 9001 checklist to help you tick off your clause-by-clause requirements.
Some important initial steps to focus on include:
Clauses 1 & 4: Scope & context
1. Define the context of your business with a SWOT and PESTLE analysis
2. Identify, monitor and review internal and external factors that impact the business, including your interested parties
3. Define who and what is relevant to the QMS
4. Use this information to set the scope and boundaries of your QMS
5. Plan, control and operate your QMS processes – with mechanisms for measuring, maintaining and improving them
6. Document plans, procedures, checklists, processes
7. Demonstrate planned process operations align with actual results
Clause 5: Leadership
1. Clearly organize and structure your operational hierarchy with clear roles and responsibilities
2. Demonstrate leadership commitment to quality
3. Ensure customer, statutory and regulatory requirements are understood, systematized and met
4. Communicate quality policy and QMS requirements
5. Identify vulnerabilities and issues
6. Enhance customer satisfaction
7. Set and review short-, medium- and long-term objectives
Clause 7: Support
1. Control, measure and monitor infrastructure and equipment
2. Capture, preserve and maintain internal knowledge
3. Identify and fix competency deficiencies
4. Communicate to internal and external stakeholders the consequences of non-conformance with the QMS
5. Control the creation and updating of information streams, including documentation
Documentation requirements
It's absolutely essential for your ISO 9001 compliance that a clear, effective document management system is in place to help you create, maintain and distribute information with confidence.
You'll need to effectively document every process in your business, with both planned and actual performance.
Document requirements include:
- Plans
- Procedures
- Checklists
- Designs
- Evidence
A holistic, well-documented QMS should cover most or all of the elements here:
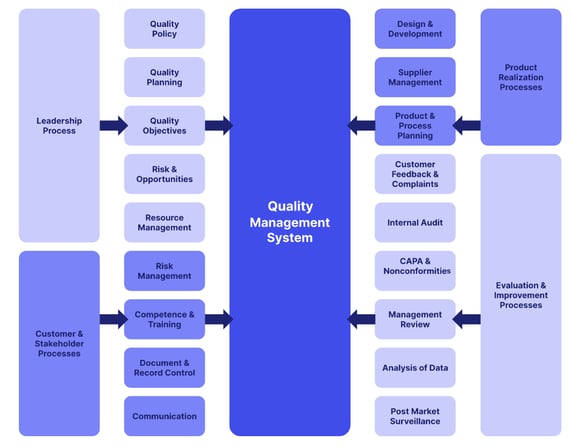
FURTHER READING:
Training & employee awareness
An effective ISO 9001 QMS needs support and participation from a well-trained and connected workforce.
An IDC whitepaper, 'Counting the Cost of Employee Misunderstanding', found that 89% of life science companies have experienced unplanned downtime from suboptimal training and employee misunderstanding.
85% of those businesses saw reputational damage as a result, and over a third lost business. This is the exact opposite of the proactive, controlled, customer-first quality focus enshrined in ISO 9001.
Get a mechanism in place for training staff on your QMS processes and confirming understanding.
Check the training completion rate for each and every process.
And ensure training records are maintained with integrity like any other QMS documentation - your ISO 9001 auditor will want to see evidence that your staff are trained and competent to execute their roles!
Training and developing your team is every bit as important as training your leaders. Have clear and complete procedural information readily available, mandate training, and provide your team with all necessary educational materials they need to complete it.
Your company culture should encourage them to ask questions and to seek clarification when necessary. Your team should be comfortable sharing their concerns, so you can always be improving!
FURTHER READING: The risks of ISO 9001 non-conformance
ISO 9001 certification
ISO 9001 certification, as we saw at the beginning of this article, is incredibly popular.
Millions of businesses across the planet have secured ISO 9001 certification as testament to their customer focus and their maintenance of a functional QMS.
How will your business benefit from securing certification?
Benefits of ISO 9001 certification
ISO 9001 adherence brings a string of powerful benefits to your organization, including:
1. Bringing quality and continuous improvement into the heart of your business
2. Aligning your business around documented, repeatable processes
3. Securing leadership commitment to quality
4. Harnessing improvement opportunities as well as controlling risks
5. Establishing a business-wide governance and quality tool
6. Boosting profitability and customer satisfaction, in turn slicing churn
7. Building an integrated regulatory approach through Annex SL as a springboard for other compliance with other quality standards
ISO 9001 compliance proves to the world that your company, well... works.
You've got control of your processes.
You've anticipated and controlled your risks.
And you're constantly measuring and optimizing your ways of working to maximize how happy your customers are with your products and services.
What's not to like?
And if you're struggling to get senior management to engage with or care about ISO 9001, remind them of the potent financial benefits.
Because your ISO 9001 QMS is all about fixing risks and wastage, compliance naturally brings leaner and more efficient ways of working that can save thousands, tens of thousands, or even hundreds of thousands of dollars.
And automating your QMS with a digital electronic quality management system (eQMS) only compounds these benefits.
Maintaining ISO 9001 compliance
This doesn't mean ISO 9001 compliance is easy.
It demands constant upkeep, and the establishment of consistent processes that output repeatable products and services.
You'll need to get a few key operational elements in place to maintain compliance.
Internal audits
Like any ISO standard, the best way to get ready for a real third-party audit is to complete dry-run practice audits internally.
Internal audits allow you to find and address weaknesses and gaps in your ISO 9001 compliance.
Our ISO 9001 checklist is a good way to identify the areas to check up in your internal audit cadence.
Some other broad questions to structure your initial internal audits around include:
- Have we determined the external and internal issues that are relevant to our business and its strategic direction?
- Have we determined the inputs required and outputs expected from our QMS processes?
- Have we determined the knowledge necessary for the operation of our processes?
- Does the QMS scope exist as documented information? Are exclusions recorded and justified?
- How are top management demonstrating a hands-on approach to the management of our QMS?
- How are we applying a systematic methodology for consistently and effectively determining risks and opportunities?
Management review meetings
As we've seen, your leadership team need to show responsibility for, and active engagement with, the day-to-day running of your QMS and adjoining business processes.
Management review sessions are your opportunity for your exec team to review the suitability and effectiveness of your QMS and take documented steps towards continuous improvement.
Reviews should take place at least once a year and include:
- Presentation and analysis of relevant QMS data, particularly how it relates to topline business objectives and the overarching ISO 9001 target of customer satisfaction
- Agreed action points and improvement steps to take
- Review of action points from previous session
Continual improvement
We've seen how Clause 10 of ISO 9001 focuses solely on improvement.
An 'As-Is' approach isn't explicitly mandated in ISO 9001, but it's a good way to think about and structure your continual improvement activities:
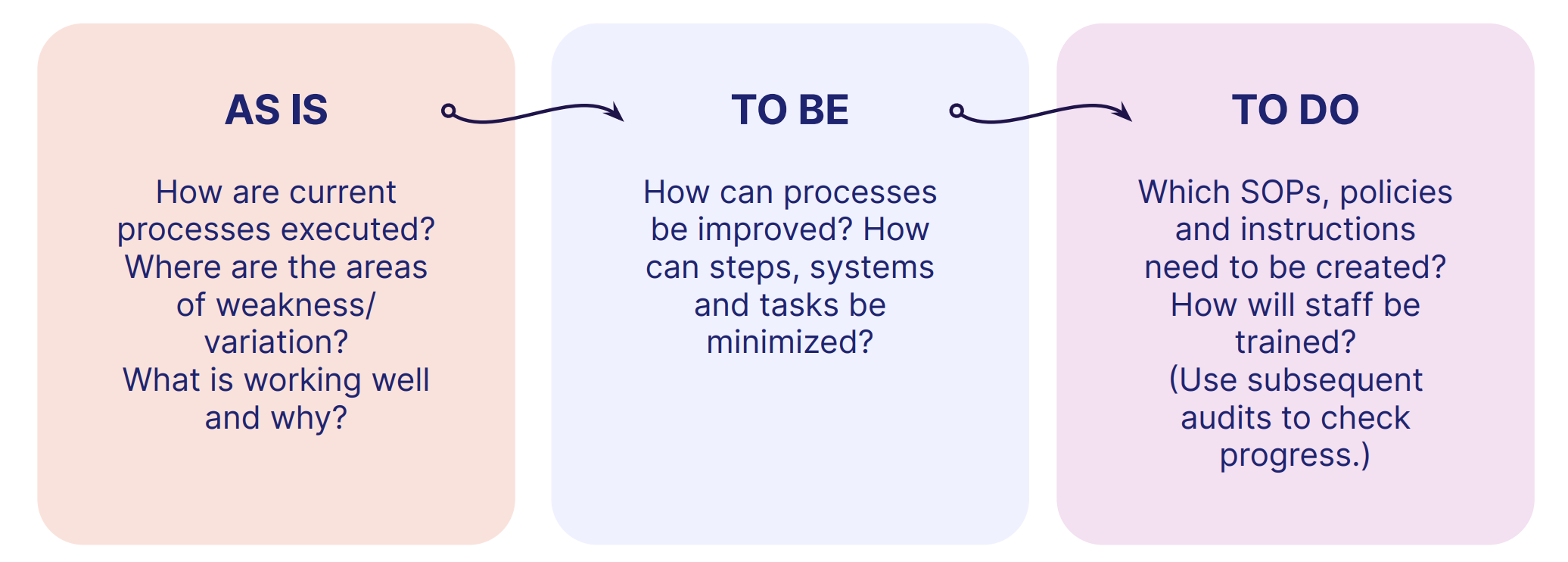
Surveillance audits and recertification
After securing ISO 9001 certification, light surveillance audits will be executed annually by your certification body to ensure your organization is effectively maintaining compliance.
More comprehensive recertification audits take place every 3 years or so, with fresh certification issued after successful completion.
ISO 9001 and other ISO standards
The beauty of ISO 9001 is its broad-stroke fundamental nature.
It's the best springboard possible for getting a robust and operational QMS in place, that you can then adapt and grow for additional compliance with other industry-specific standards such as ISO 13485 for medical devices.
ISO 9001 vs ISO 13485
Medical device companies often turn to ISO 9001 as a first step towards more niche ISO 13485 compliance.
It's important to understand the areas of crossover and difference between the two if your business plans to follow this approach.
Both standards help organizations achieve a quality management system. Both place a focus on risk mitigation and assessment. Both utilize the Shewhart cycle, also known as Plan Do Check Act. They each place a focus on building competency and a suitable infrastructure for quality management, and both emphasize understanding and prioritizing the needs of your customer for the realization of quality products.
However, since ISO 13485 is a specific requirement for a higher-risk regulated product, namely medical devices, it's obvious that it includes additional requirements which go beyond the scope of ISO 9001.
Read the complete list of differences between ISO 9001 and ISO 13485 here
Achieving long-term success with ISO 9001
ISO 9001 is the start of your organization's quality journey.
An effective QMS, built in line with the 9001 model, is your pathway to a stronger, leaner, sharper and more competitive business.
Download your ISO 9001 checklist to start working your way towards compliance.
