What is an ICH Q10 pharmaceutical quality system?
ICH Q10 is a crucial quality management standard for pharmaceutical companies.
ICH Q10 was developed by the International Council on Harmonization (ICH) to provide a comprehensive framework for the modern pharmaceutical quality management system.
How do you interpret the standard's myriad requirements and get an ICH Q10 pharmaceutical quality system in place? This comprehensive guide breaks down what you need to know.
Table of Contents
- What is a pharmaceutical quality system?
- Why do you need an ICH Q10 pharmaceutical quality system?
- The 4 stages of the ICH Q10 pharmaceutical lifecycle
- The 5 clauses of ICH Q10
- 7 top tips for a world-class ICH Q10 pharma quality system
- 5 ICH Q10 myths
- Embed a digital ICH Q10 pharmaceutical quality system that maximizes your success
What is a pharmaceutical quality system?
Like any regulated company, pharma and biotech organizations require a robust quality management system (QMS) to ensure their lifesaving products are safe and effective.
But a generic, unfocused QMS isn't enough in such a niche, highly regulated vertical. A targeted and industry-specific pharmaceutical quality system (PQS) is therefore crucial.
ICH Q10 is a single comprehensive model offering the structure and guidelines necessary to build an effective pharmaceutical quality system that supports development and manufacturing across the drug lifecycle.
The ICH Q10 pharmaceutical quality system guidelines are applicable to any company that manufactures pharmaceutical drug substances and drug products, including biotech and biological products.
The guidelines are based on ISO quality concepts and GMP regulations, and form one part of a tripartite structure alongside ICH Q8 (pharmaceutical development) and ICH Q9 (quality risk management).
Since GMP doesn’t cover the entire pharmaceutical development lifecycle, or fully address the quality processes you need to bring your product to market and keep it there, ICH Q10 marries GMP with the ISO 9001 structure of management responsibility and continuous QMS improvement to offer a blueprint for a robust and modern pharmaceutical quality system that looks like this:
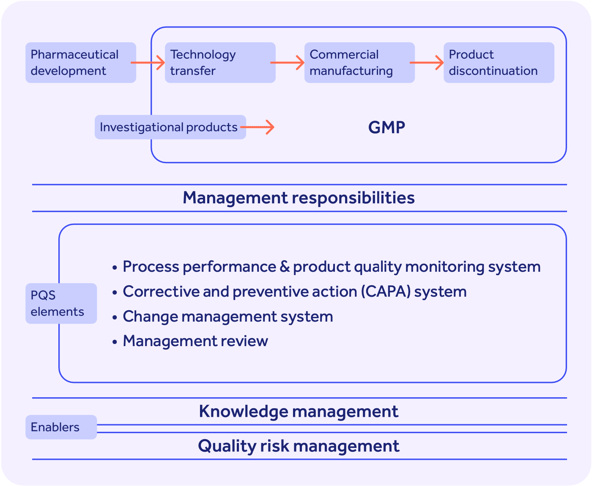
It's not intended to create new expectations beyond the ones currently required by regulators, and so its extra components form an optional standard not mandated for market access in any national territory.
That doesn't mean you should ignore ICH Q10 compliance, though.
Building an effective, holistic and controlled pharmaceutical quality system with ICH Q10 compliance positions your pharma or biotech business for long-term, quality-centric success.
FURTHER READING:
What is cGMP in the pharmaceutical industry? An expert round-up
Why do you need an ICH Q10 pharmaceutical quality system?
In 2001, a PWC report uncovered shocking levels of inefficiency and waste
in the pharmaceutical world, with scrap and rework rates touching 10% and
costs of poor quality exceeding 20%.
In 2003, FDA Commissioner Mark McClellan issued a damning assessment
of drug manufacturing quality in the United States, labelling pharmaceutical
quality management as less developed than that of potato chip and soap
manufacturers.
And in 2005, an IBM report found the average pharmaceutical process had an average sigma level of 4σ. Pushing processes closer to 6σ with a robust QMS driven by continuous improvement and quality by design (QbD), the report suggested, could unlock cost savings of over $10 billion a year.
Two decades later, the picture is better – but not dramatically so.
Recalls, wastage and suboptimal quality continue to threaten pharmaceutical businesses and suppress the potential of start-ups and scale-ups in the sector.
In 2018, the FDA Drug Shortage Task Force launched an investigative analysis of historic drug shortages. 31 senators and 104 representatives had asked for help after a steady growth in drug shortages across the US, with some shortages lasting 8 years or more.
The FDA summarized the DSTF’s findings with some recommendations in a 2019 report. Their main takeaway was:
Many pharmaceutical manufacturing firms have focused their efforts on compliance with cGMPs, which include standards for material systems, equipment and facilities, production, laboratory, packaging and labeling, and a quality system.
These standards, however, are foundational and set a minimum threshold that companies must achieve in order to be allowed to supply the U.S. marketplace. They do not include more advanced levels of quality management...
It's clear that solely meeting the baseline of cGMP is holding back pharmaceutical organizations, and the problem isn’t just an American one: general lack of a continuously improving, training-driven QMS has now been the primary finding in UK MHRA pharmaceutical inspections for the past 5 years in a row.
Luckily, the industry is taking steps to reward pharmaceutical quality more than ever. The FDA's so-called Quality Management Maturity program got off the ground in 2022, and proposes objective quality 'scores' of pharma and biotech companies to help supply chain actors source quality-centric partners.
Good pharma quality practice, going beyond the bare minimum of cGMP, will be acknowledged, recognized and promoted.
The time's therefore never been better to embrace the strongest possible quality framework for your pharma operation - and ICH Q10 is your blueprint to do so.
Complying with ICH Q10 lowers your costs, sharpens your processes, signals trust, strengthens your pharmaceutical business, and will maximize your QMM score when the program goes live!
Watch our webinar explaining the Quality Management Maturity program
The 4 stages of the ICH Q10 pharmaceutical lifecycle
ICH Q10 is designed to be implemented through all four stages of your pharmaceutical product lifecycle, as follows:
1. Pharmaceutical development
The goal of the pharmaceutical development phase is to design your product and plan its manufacturing process.
The development process should be able to map out a route to production that delivers the necessary drug performance, meets the needs of patients and healthcare professionals, and complies with your relevant regulatory guidelines.
Key technical activities at this stage include:
- Development of drug substances
- Development of formulations, including container and closure systems
- Manufacturing investigational products
- Development of a delivery system (when relevant)
- Development of the manufacturing process and planning the scale-up
- Development of an analytical method
2. Technology transfer
Activities related to technology transfer are intended to transfer process and product knowledge between manufacturing and development.
This knowledge is the foundation for the manufacturing process, your process validation approach, your control strategy and your ongoing continual improvement.
Key activities at this stage include:
- Transferring products from development to manufacturing
- Transferring products within or between the manufacturing process and the testing sites for the marketed products
RELATED READING: The key to future-proofing pharma compliance
3. Commercial manufacturing
Manufacturing activities are designed to achieve product realization by establishing and maintaining a state of control and enabling continual improvement.
Your ICH Q10 PQS should ensure that product quality is fulfilled, suitable process performance is achieved, controls are appropriate, improvement opportunities and identified and evaluated, and knowledge is continually expanded.
Key activities at this stage include:
- Acquisition/control of materials
- Provisioning of facilities, equipment and utilities
- Manufacture of products, packaging and labels
- Quality control and assurance
- Release, storage and distribution of product
4. Product discontinuation
Product discontinuation activities are designed to manage the end of your product's lifecycle. The discontinuation process should be planned well ahead of time, then used to remove your product from usage with control and full visibility.
Key activities at this stage include:
- Document retention
- Sample retention
- Continuous product assessment and reporting
The 5 clauses of ICH Q10
Now we've seen how your ICH Q10 pharmaceutical quality system should touch your product's end-to-end lifecycle, let's dive into what the standard actually prescribes.
There are 5 clauses to the ICH Q10 standard, but since the fifth is just the glossary of key terms, the first 4 clauses are the primary areas to scour to understand how to embed compliance.
1. PQS
The whole point of the ICH Q10 standard: building a holistic pharmaceutical quality system, or PQS.
Your ICH Q10 PQS should enable 4 key things:
- Process performance and product quality monitoring
- Corrective and preventive action (CAPA)
- Change management
- Management review
In doing so, your PQS should empower your business to consistently provide pharmaceuticals that meet patient needs and your applicable statutory and regulatory requirements.
You should have effective monitoring and control systems to assess and maintain process performance and product quality.
Management should be committed and engaged, with continuous improvement as your objective - not baseline cGMP compliance (see above!)
And importantly, ICH Q10 makes reference to the ‘enablers’ of knowledge and risk management, which should be maximized internally to optimize product quality and patient safety.
That means a culture of shared knowledge and proactive risk management, with both feedback and feedforward underscored by frequent management reviews.
FURTHER READING:
2. Management responsibility
In line with that ISO 9001 cross-fertilization we discussed above, the second clause of ICH Q10 breaks down the areas that top management
should demonstrate commitment to.
Your senior leaders should show active involvement with the quality agenda, including:
- Allocation of resources
- PQS participation and oversight
- Communication
- Policies, planning and frequent review
3. Continual improvement of process performance and product quality
We saw above how industry initiatives like the QMM program aim to reward pharmaceutical companies that don't rest on the laurels of cGMP compliance.
ICH Q10 mandates that you get systems in place to continuously test, control and optimize your products and processes, so your business is never standing still.
You can do so with elements like:
- A CAPA system
- A control strategy
- Feedback capture
- Change management
4. Continuous improvement of the PQS
In keeping with the theme of continuous improvement, your PQS itself should also be continuously optimized.
ICH Q10 mandates a formal structure for reviewing and continuously improving the operation of your PQS, including:
- Monitoring of internal and external impact factors
- KPI assessment
- Management review outcomes
- Monitoring of PQS objectives
5. Glossary
As explained above, the glossary simply defines some key ICH Q10 terminology, like 'design space', 'state of control' and 'enabler'.
7 top tips for a world-class ICH Q10 pharmaceutical quality system
1. Look to Pharmaceutical Inspection Cooperation Scheme (PIC/S) guidance for some of the broader and more ambiguous areas of ICH Q10, such as ‘demonstrating the effectiveness’ of your PQS
2. Weave GxP into your business operation. Roughly speaking, an ICH Q10 pharmaceutical quality system combines the requirements of cGMP with the traditional QMS structure of ISO 9001.
Following cGMP guidelines within an integrated QMS is the backbone of your ICH Q10 compliance - but don’t stop there.
Embed as many GxP processes as are applicable and appropriate for your business. Alongside GMP, consider GLP if you have a laboratory, GDocP for your document stack, GVP for your post-market surveillance activities, GDP for your product distribution, and so on.
Use our GxP toolkit for more help!
3. Keep the ‘4 pillars’ and ‘4 lifecycle stages’ front and center of your ICH Q10 quality planning and let them structure everything you do
4. The content of Q10 which goes beyond GMP guidelines is technically optional – but embrace these areas as key competitive differentiators. A world class PQS will bring GMP automatically in its wake!
5. The ‘desired state’ referred to by the FDA’s Dr Woodcock gives you a flavor of what you need to do to comply:
‘A maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high quality drug products without extensive regulatory oversight’
6. Continual quality improvement is at the core of Q10 – consider how to dedicate as much of your time to this as possible by digitizing and automating admin and quality control/assurance work
7. Think of ICH Q8/9 as supporting elements of your ICH Q10 PQS
5 ICH Q10 myths
Like any complex quality management standard, ICH Q10 is prone to misunderstanding and misinterpretation.
Here are 5 of the most common myths, and the truth behind them!
1. "You can get certified to ICH Q10."
Just like ISO 14971 for medical devices, ICH Q10 is sometimes misinterpreted as a certifiable quality standard like ISO 9001.
In fact, it’s an optional guideline that gives your business the ingredients for a functional pharmaceutical QMS and makes you far more likely to satisfy FDA, MHRA and EU auditors and get your products to market.
2. "Pharma businesses with outsourced operations don’t need a full ICH Q10 PQS."
ICH Q10 places emphasis on site-by-site compliance.
Even if the sites don’t belong to your business, the responsibility nevertheless rests on you as the sponsor to monitor, assess and guarantee quality.
Clause 2.7 focuses specifically on management of outsourced activities and purchased materials for this reason.
3. "Paper-based quality systems are suitable for long-term ICH Q10 compliance."
You can get by with a paper-based system in the short term if your company is very small.
But if you have any growth ambitions, long-term go-to-market plans, or desire to maintain a baseline of compliance without undue effort, a paper-based system is unsuitable.
More and more pharma and therapeutic companies are turning to electronic quality software as a way to ease the burden of compliance and make their way to the optimized Six Sigma processes called for by the industry.
4. "You need ICH Q8 and Q9 before you can work to Q10."
It’s best to have all three elements supporting and feeding into each other, and the ICH explicitly refers to the ‘tripartite’ approach.
But ICH Q8, 9 and 10 aren’t strictly integrated and there’s no set order for your PQS pathway. For instance, you can still start building and applying the core elements of ICH Q10, like CAPAs and knowledge management, even if you don’t have a complete quality by design (QbD) or risk management approach yet.
Start with the elements you already have in your business, refine and strengthen them, then bring in the additional elements you need.
5. "ICH Q10 is only for pharmaceutical companies, not biotech companies."
Pharmaceutical and biotech businesses are technically different: pharmaceutical companies produce synthetic small-molecule drugs which require a New Drug Application (NDA) with the FDA, while biotech businesses make cellular and biomolecular products using living biological samples, which require a Biologic License Application (BLA).
But although ICH Q10 pertains to a ‘pharmaceutical quality system’, its requirements and demands are just as applicable to a biotech company as a pharmaceutical one.
We therefore recommend pursuing ICH Q10 compliance whether your company works with synthetic or biological products – your company will be regulated and audited in the same way!
FURTHER READING: Biotech vs. pharma: differences and similarities
Embed a digital ICH Q10 pharmaceutical quality system that maximizes your success
Manual paper-based quality systems bog down pharmaceutical quality managers in time-intensive admin tasks and block the continual improvement, knowledge management and 20/20 visibility demanded by ICH Q10.
Electronic quality management systems designed for life sciences companies — like Qualio — are designed to embed the quality control, operational efficiency, connected knowledge, regulatory compliance and robust manufacturing processes you need for an ICH Q10-compliant PQS.
The perfect eQMS should provide essential functions such as document control, training and CAPA management, while fostering collaboration and eliminating and automating quality management tasks.
When you implement a robust eQMS like Qualio for your pharmaceutical company, you’re instantly one step closer to building – and maintaining – a compliant and effective ICH Q10 pharmaceutical quality system.
Book a demo with us to see us in action!
