The hidden costs of a free quality management system
Regulated businesses in the earliest stages of their lives often have little disposable capital, so it can be tempting to turn to a free quality management system option to tackle those unavoidable quality and compliance obligations.
Using free quality management tools like paper and email, or even adapting tools like SharePoint into a kind of free quality management software platform, might seem like a good way to minimize expenditure.
But these options bring a slew of hidden costs, risks and icebergs to think about.
Here's why a 'free' quality management system is anything but, and why the strongest, most quality-centric and risk-focused organizations on Earth all invest money into their quality systems.
Table of Contents
Paper
Paper is the king of cut-price quality management.
It's everywhere, it's easy-to-use, and it gives the illusion of a 'free' tool. Since your organization probably has a store of paper on-site anyway, it's logical to assume that simply adopting some of it to manage your quality management system (QMS) processes is a free pathway to quality and compliance.
If your organization has 1, 2 or maybe 3 heads, paper could be somewhat viable. The relatively small number of procedures, records, forms and quality documentation flowing through your company means a filing cabinet or two might house your QMS comfortably enough.
But the question is: do you want your company to remain that size forever? And is this approach really 'free'?
Let's take a closer look.
A paper-based, free quality management system will naturally begin to creak as new data accumulates and headcount grows.
Paper demands physical sourcing and traceability, meaning that as your company grows, your employees spend more and more time looking for the vital QMS information they need to do their jobs.
In fact, AIIM estimates workers with paper-based document management systems spend half an hour each day just searching for the information they need to do their jobs.
That’s 2.5 hours every single week lost to unproductive searching. By the IDC's calculations, it could be even more.
None of this takes into account the hidden labor costs that come with a paper-based free quality management system.
A Coopers & Lybrand study found that:
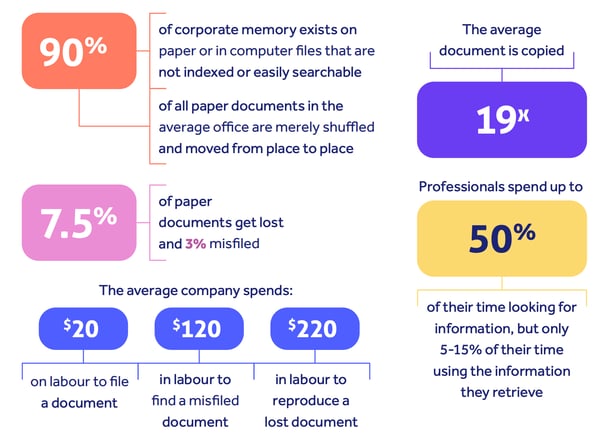
Add to that time spent on unavoidable, non-value-adding paper tasks like filing and retrieval, duplication of effort as paper records are transcribed into reporting systems, and locating and recreating lost documents, and paper brings an undeniable loss of productivity and efficiency once your business manages any kind of significant volume of information.
Even small organizations with a heavily paper-oriented operation spend an average minimum of $25,000 every year on paper production and usage costs.
This rises to an eye-watering $175,000 as you scale.
And a single filing cabinet costs $25,000 to fill and $2,000 a year to maintain.
Paper, then, isn't really free at all.
Not only that, managing any kind of regulated life science product with it can have disastrous consequences.
We collected 4 scary, anonymized and completely true quality management horror stories in 2023.
One medical device quality manager, who we'll call 'Andy', faced a nightmare month of 80-hour-week stress after a deadly device fault could only be traced by poring over stacks of disconnected paper design control records spread across multiple archives.
A multimillion-dollar recall ensued, and might have been avoided had a robust quality system underpinned by digitally traceable design controls been in place.
So much for a free quality management system.
And these aren't the only ways paper inflicts a heavy price on your quality system.
Because paper demands constant and ever-growing admin, upkeep, filing and signing, it also saps energy and momentum from profit-making commercialization projects.
Ditching paper with a dedicated eQMS tool can therefore trigger a considerable boost to your long-term profitability by accelerating the milestones that make your company money - as this medical device case study snippet shows:
Because paper is so often integral to a free quality management system, these very real problems and challenges demonstrate the hidden costs you need to consider before you turn to a 'free' QMS.
Now, let's look at email.
Can email act as a completely free tool to manage your QMS?
After all, it's digital. Account membership is free. Emails don't require filing cabinets or complex admin upkeep.
But it, too, brings considerable hidden costs and challenges that damage your quality, compliance and audit readiness.
Long email chains, studded with back-and-forth document attachments and uncontrolled document versions, are an almost universal occurrence at businesses with a free quality management system in place.
Unlike paper, these practices really are cheap to run.
Yet they make it difficult, or impossible, to place your finger on the key touchpoints of your quality management processes.
“When was this document version superseded?”
“How was this defect from Q2 last year addressed with a CAPA?”
“What role did this quality engineer play in refining Version 3.1 of this SOP?”
Any auditor will ask these types of questions as they interrogate your free quality management system.
Answering them by attempting to unearth emails from cluttered, uncontrolled inboxes and folders isn’t a good look, and is a fast track to a series of costly non-conformances in your audit findings.
Email, in practice, delivers the same results as running to the filing cabinet to rifle through reams of paper or pulling documents from an uncontrolled Dropbox folder: complicated, weakened and untraceable quality and compliance integrity.
The hidden costs of this can be substantial. Audit failure slows your route to market and pushes back your profitability horizon in those crucial early days.
And if you're already marketing a regulated product, patchy email-based processes in a free quality management system can trigger a recall or fine as compliance slips and issues emerge.
Email might be more cost-effective to run day-to-day than paper, but it brings the same hidden costs and risks.
How about a free quality management software system like SharePoint?
SharePoint
Your quality management system needs a stack of controlled, organized documents underpinning everything.
So on the list of free quality management system software options that businesses turn to, document repository SharePoint is often near the top.
It's a free tool that comes with the Office365 subscription your business probably already has.
It allows configurable folder structures to house quality documents in a taxonomy and hierarchy that you choose.
And it offers a digital, password-protected, cloud-based access point that anyone in your business can use to view key QMS information.
What's not to like?
From a quality and regulatory perspective, quite a few things.
Let's look at what SharePoint doesn't do.
It has no built-in e-signature functionality - a capability that's required for compliance with modern electronic record regulations like FDA 21 CFR Part 11 or EU Annex 11.
Further investment in third-party e-signature software is required to meet this requirement. If you're a regulated business, that's a hidden cost that can't be sidestepped.
The same can be said for validation. Any software system used by a regulated organization, like a life science company, must pass through a validation process to ensure it's compliant and fit for purpose in your environment.
Dedicated quality management software from any reputable vendor will include validation, or even more modern computerized system assurance, as part of your service. SharePoint, on the other hand, isn't designed for regulated GxP environments - meaning responsibility for those validation tasks falls to you, not Microsoft.
That means validating your SharePoint system, revalidating it whenever it's updated, and validating any add-ons like that e-signature functionality.
That means considerable time spent by your team, or money invested in a consultant to help you. That's another hidden cost.
And because it's not a dedicated quality tool, SharePoint also means that the vital documents driving your free quality management system have no version control, change histories or overarching audit trail functionality baked into them.
This traceability is a core requirement for modern compliance. Without it, your free quality management system can become peppered with multiple versions of the same document without any auditable timeline of activity.
Compliance with modern benchmarks like GDocP and ALCOA+ becomes impossible, and when an auditor comes knocking your inability to confidently say when a document version superseded another, or when a key update took place, will bring a massive red cross on your report.
As above, this quality, compliance and integrity weakness invites substantial hidden costs - from delaying marketization to inviting regulatory punishment.
Hear a quality director explain why you should never use SharePoint as part of a free quality management system:
Lastly, SharePoint doesn't allow the kind of fast, intuitive document collaboration that a modern regulated company needs to build and launch compliant QMS documents.
That means you'll need to either invest in a tool with dedicated collaboration functionality, like inline commenting and approval workflows, or manage these processes manually - bringing us back to the email daisy-chains we touched on above.
Free quality management isn't free
Quality is crucial to your company's operation.
As such, scraping by with a home-brewed free quality management system isn't the good idea it might initially seem.
Hidden costs abound, from admin and upkeep demands to the dramatically increased risks of quality issues and compliance slippage.
Free quality management software options like SharePoint are neither free or dedicated quality tools, and they therefore bring their own problems and hurdles with them.
There's a reason that all quality-centric companies spend money on their quality management systems. Your initial outlay might be higher than sticking with paper or email, but the advantages and the return on investment of a dedicated tool far outweigh the costs.
Free quality management software isn't free. And by the same token, paying for a dedicated eQMS platform now will save your company considerable money, time and headaches long into the future.
Book a demo of Qualio to see how our eQMS makes quality the core of your organization.
