CAPA management software: benefits, top features, and how to select the best
CAPA software is now a must-have for regulated companies in the 21st century. In the life science industry, for instance, your regulators will require, as part of your go-to-market quality activities, that your company has a strong formal process for managing corrective and preventive actions (CAPAs).
This includes identifying problems, conducting root cause analysis, implementing solutions, and verifying that the original problem has been properly resolved. To manage this process effectively and ease compliance burden, forward-thinking companies are embracing CAPA software.
Choosing the best CAPA software is important to ensure that your company isn't at regulatory risk and can address its quality issues effectively.
Here's what you need to know about corrective and preventive actions software and the functionality that's essential in the best CAPA management software for your business needs.
What is CAPA software?
CAPA software is a specialized application that helps organizations manage their corrective and preventive actions digitally.
Also known as corrective and preventive actions software, or even just corrective actions software, CAPA software systems are designed to simplify the process of complying with regulatory requirements for CAPA processes.
CAPA management software typically includes features and functionality to support, guide and document the entire CAPA process from identification of issues to verification of effectiveness and close-out.
And crucially, the best CAPA software allows organizations to ditch clunky and time-consuming paper and spreadsheet processes and unite their CAPA activities in a single traceable area - often in the cloud.
What is a CAPA used for?
CAPAs cause more compliance trouble for life science companies than any other area of quality management. Year after year, lax CAPA management is a key trigger of Form 483 submissions and warning letters for medical device manufacturers.
It's no wonder - life science quality management and CAPA execution is a complex, multi-person process that's often difficult to coordinate, and regulated industries like medical devices have a series of stringent CAPA requirements that auditors will be on the lookout for.
It’s clear that organizations need to brush up on and optimize their CAPAs.
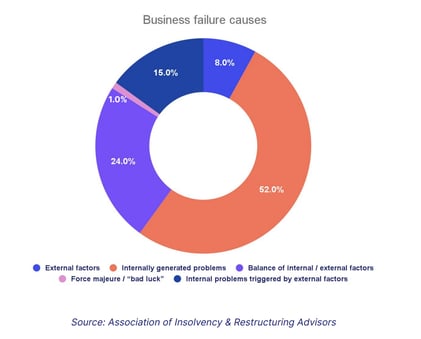
And since studies show over half of business failures are self-inflicted by internally generated issues, CAPAs can be critical to keep your business alive and kicking.
The role of corrective and preventive actions (CAPA)
CAPAs are, in short, a way to address and permanently fix problems that emerge in how your company operates.
From addressing manufacturing weaknesses and product defects to ironing out inefficiencies and stamping out repeat problems, strong CAPAs are your gateway to continuous improvement.
As the name suggests, CAPAs are really a double action of 'correcting' a problem then 'preventing' its reoccurrence:
-
The corrective action involves identifying and addressing the root cause of a problem or non-conformance that has already occurred, then taking actions to correct it.
-
Preventive actions involve identifying potential issues, risks, or areas for improvement before they occur, implementing continuous measures to prevent them from happening, and consistently monitoring processes to ensure effectiveness.
Regulators are increasingly pushing for life science companies to do more of the latter, and less of the former.
After all, being able to proactively identify potential problems and prevent them happening is cheaper, faster and more effective than reacting to existing fires: the '1-10-100' quality rule states that $1 properly spent on defect prevention is equivalent to $10 spent on product checks and $100 spent on fixing failures.
CAPA processes typically involve steps such as:
- Identification of the issue or non-conformance
- Investigation to determine the root cause(s)
- Development and implementation of an action plan
- Monitoring and verification of the effectiveness of the actions taken
- Documentation and reporting of the entire end-to-end CAPA process to prove that the action took place and to give it clear audit traceability
Effective CAPA systems are crucial for maintaining your product and process quality, and you'll need to show you have an effective CAPA system in place for compliance with pretty much any modern quality management standard.
Choosing the best CAPA software
Investing in a long-term corrective action request software platform from a reputable vendor is usually worth the money when it comes to CAPA management, since the need for documenting quality events and corrective and preventative actions will remain a key requirement throughout your product lifecycle.
It's important to remember that not all corrective action software is created equal. You'll want to consider a number of factors before making a decision, including ease of use, regulatory compliance, scalability, and customer support—in addition to price.
To provide effective safeguards against regulatory risk, corrective actions software is generally implemented as a module or section within a broader electronic quality management system (eQMS). This holistic approach allows integration of CAPA software processes with those for audits, training, documents, suppliers and so on.
How do you choose the best CAPA system software for your company?
We'll review the key functions you should look out for.
Top features of CAPA software
The FDA offers minimal guidance for selecting the best CAPA software, aside from referring organizations to consider cGMP requirements and CAPA software which allows a quick response to customer complaints.
FDA Postmarket and Consumer Branch Chief Joseph Tartar recommended that:
“Manufacturers should consider [if] their corrective action and preventive action documentation can demonstrate to the FDA that the manufacturer’s quality system is effective and enables the manufacturer to identify problems quickly and implement effective corrective and preventive actions.”
At a high level, your CAPA software must:
- Allow collaborative CAPA procedures which address quality system requirements
- Support tagging and categorization to organize and track the sources of product quality concerns
- Contain analytics to monitor trends for preventive action
- Integrate with surrounding systems and QA processes to assure data quality and 'pull in' problems to fix
- Facilitate statistical analysis and formal failure investigations
- Allow you to validate the success of preventive or corrective actions
While these requirements are useful, they don’t help you identify which CAPA software options are likely to fail in a real-world implementation.
Fortunately, by understanding what a good CAPA process looks like, you can identify the following key capabilities.
RELATED READING: Identifying the 4 most common problems with your CAPA process
CAPA reporting
Any corrective action software that you choose needs to deliver powerful reporting — after all, that’s the primary reason to leave paper trails behind.
Corrective action database software will help you achieve true end-to-end traceability across your product lifecycle. You can see links between records, close the loop on your process, and enjoy near-instant access to all of your CAPA data when you need it.
You won’t have to worry about building a manual paper-based report and realizing too late that you didn’t have all the information because someone forgot to put a page in the binder.
Any CAPA management software needs to offer basic functionality for quality event and CAPA reporting. This is defined as a standardized report template for recording defects or complaints and the steps taken to correct an issue. At a minimum, your organization's CAPA report should include details such as:
- Issue description
- Action taken
- Resolution
- Resources and team members
- Planned resolution date
- Actual resolution date
Documenting these basic characteristics of your CAPA program is a bare minimum component of complying with FDA cGMP requirements. However, basic CAPA capabilities which provide evidence in case of an audit isn’t usually enough.
Ineffective reporting is among the top reasons CAPA software fails to protect organizations from true risks such as poor product quality or waste.
If your corrective and preventive actions software produces reports which are little more than spreadsheets or documents, it can quickly become a catch-all for user-generated data. These reports can build up in your CAPA software system. Unless you have someone specifically dedicated to managing CAPAs, these reports can be overlooked or forgotten. Issues may never be effectively resolved, which can spiral into costly quality concerns.
Perhaps most importantly, basic reporting capabilities don’t allow your organization to filter or understand trends. In addition to documentation, your reporting should offer insight into emerging issues so you can take preventive action, and understand when small data points are telling a larger story. While small issues should be handled as non-conformances, CAPA reports are an important management tool for creating process improvements.
CAPA software integrations
The best CAPA software must integrate with your other systems and technologies for quality management, or be part of a broader eQMS. If your corrective action software doesn’t integrate with surrounding systems, you’re losing opportunities for automation and opening yourself up to errors by entering CAPA data manually.
Qualio's Salesforce integration, for instance, allows customer issues and problems to be pulled straight from our customers' CRMs into their eQMS for investigation and resolution.
Most importantly, CAPA management that doesn’t integrate with surrounding QMS systems can easily cause your organization to lose track of CAPA timelines. The single biggest advantage of corrective and preventive actions software that's fully integrated with your eQMS is organization-wide visibility.
Integrations can also lead to process improvements, including:
- Elimination of data entry or data quality concerns
- Assignment of due dates and notifications to global users
- Better communication with supply chain vendors
- Tracking trends in quality with closed-loop reporting
- Launching deviation reports or customer complaint forms linked to CAPA forms
- Ability to trigger updates to SOPs based on preventive or corrective action
Configurable CAPA forms
Strong CAPA control needs a mechanism for recording information at each stage of the process, from identifying an issue to closing it out.
Your CAPA software should therefore offer intuitive form functionality, making it easy for admin users to build and configure forms and for basic users to complete them correctly every time.
Consider whether your CAPA software system options offer robust CAPA form functionality. Capabilities to look for include:
- Web-based template editors
- Guided form workflows
- Intuitive drafting, review and approval processes
- FDA 21 CFR Part 11-compliant e-signatures for signing off actions
- Field configuration options for adding, mandating or removing form fields according to their relevance to your operation
- Corrective action request software functionality, allowing anyone in your business to quickly flag an issue and request resolution
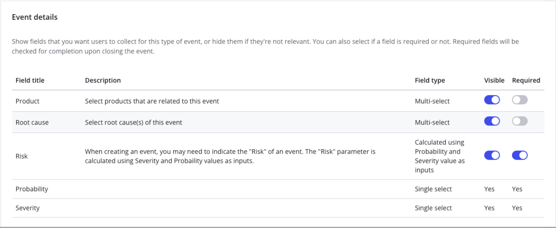 Qualio Events form set-up with collaboration and full configurability
Qualio Events form set-up with collaboration and full configurability
Linked quality processes
Too often, organizations cite 'training failures' as the root cause of an issue during CAPA investigations.
Non-conformances and complaints may be linked to human error, but training is almost never the actual root cause of an issue. Generally, there was a failure somewhere in an organization’s quality system if an individual failed to follow procedures. Perhaps they didn’t have an SOP available at the point of work. While a resulting error could be chalked up to failure, it’s more accurate to see it as a management failure to provide SOP access.
Your organization can perform more effective root cause investigations and drive better improvements if your CAPA software is intrinsically linked to other digitized quality processes. If your CAPA management software functions as a standalone system, it’s easy to treat issues as isolated incidents which can be resolved and then forgotten.
But when corrective action software is linked to your training, supplier, design control and document data, with unified analytics underpinning it all, it’s far easier to drive robust quality improvements that actually work.
Automated reminders
Bringing a complicated and life-altering drug or device to life is a big process full of multiple steps, tons of documents, lots of communication, and a whole lot of red tape.
It can be easy to forget to do things as you’re rushing to get to market.
One of the key benefits of CAPA system software is that it should make it difficult to forget or neglect issues and let problems build up in the system.
Good CAPA software should improve collaboration and help your organization avoid unnecessary meetings, emails and communication while mitigating the risk of simply missing tasks in a busy work environment.
Issues should be able to be quickly and easily assigned to automate the process of resolution, even if you’re managing a busy, globally distributed team. Software capabilities should include:
- Assignment of issues to team members
- Automated notifications
- Support for comments and collaboration
- Cloud-based access for global teams
- Mobile support for 'on-the-ground' reporting
- Workflows, which brings us in more detail to...
CAPA management software workflows
Quality teams never intend to neglect or forget issues - they simply lack the unifying tools which make it easy to effectively complete business-wide CAPA processes.
Guided workflows are crucial to a streamlined CAPA program and helping your organization close issues promptly. Consider whether your CAPA software offers configurable step-by-step guided workflows to share tasks and drive collaboration and accountability.
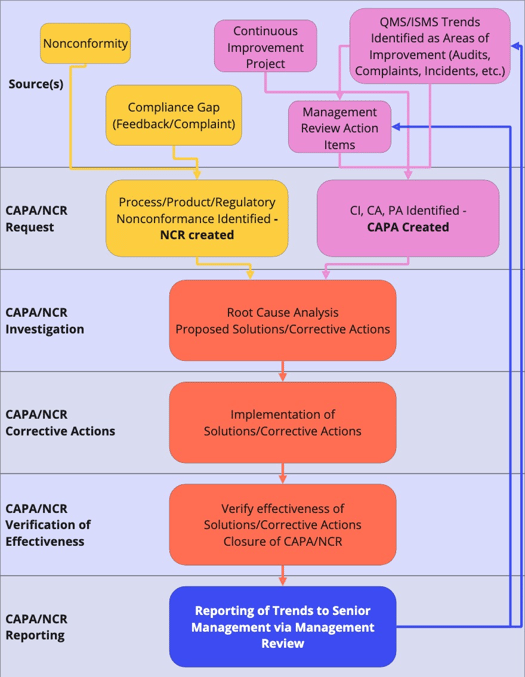 A typical CAPA software workflow. Supporting tasks can be delegated to appropriate personnel.
A typical CAPA software workflow. Supporting tasks can be delegated to appropriate personnel.
Automated data entry
Non-compliance can occur easily if your CAPA system software relies on manual data input. If CAPA-related work is tedious or time-consuming, staff members may push it aside in favor of tasks which feel more productive. In other cases, manual data input requirements can lead to data quality issues, such as inconsistency in reporting or data input errors.
Quality issues are a regulatory risk and inconsistency makes it difficult to perform effective analysis of trends. Your corrective action tracking software should facilitate process automation by pulling data from areas like:
- Supplier management
- Audit findings
- Inspection histories
- Complaints
- Design controls
Full audit trail
When evaluating CAPA software options, consider the quality of its audit trail capabilities. CAPA system software must offer sufficient audit trail requirements to make compliance natural and automatic, and to support data-driven management.
Your CAPA management system should, ideally, operate from a single place to understand how issues are linked to the entire organization and take preventive action in real-time. It’s impossible to adopt a proactive approach to preventive action with CAPA software that relies on manual data input. By using a single system for closed-loop intelligence, you can hit your regulatory requirements for audit trails and make data-informed decisions for quality operations.
Scalability
As your company grows, your software needs will too. The top platforms understand that your needs will evolve beyond just needing a place to store CAPA-related assets.
You will eventually need a tool that helps you throughout your entire product lifecycle — from early-stage ideas to interacting with active users of your devices. Today’s software vendors are continually innovating their products to add more tools and resources, streamline processes, and integrate with more of your other systems.
For example, at Qualio, we built the first web-based Quality Management System (QMS) because we knew that growing life science companies couldn’t bring life-changing devices to market if they were buried under paperwork. We knew that quality was driven by more than compliance management and complicated systems, and that good software helps companies grow instead of holding them back.
Ease of use
Like most tools — software or physical — your employees simply will not use it if it’s too hard to learn or too hard to use.
Many of the top vendors in the CAPA software space understand this and go to great lengths to ensure their software is intuitive and saves time.
If your CAPA software is a pain to use, your employees won't use it much, or they won’t use it well. But if they love it, you'll get a far better outcome and much tighter compliance with your company processes.
How to select the best CAPA software
Now we've touched on the key benefits of CAPA system software you should look for, it's important to cover how to narrow down a vendor shortlist and, eventually, pick a winner.
There are 12 key questions you should ask yourself as you scope out vendors:
1) What do I want to achieve?
2) Does it meet my industry needs?
3) Does it meet my size and scalability needs?
4) How configurable and flexible is it?
5) What's our budget?
6) How long will it take to get up and running?
7) Will it support my compliance needs?
8) Are onboarding services included?
9) What's the support like?
10) Will my quality and CAPA data be secure?
11) Will it help us go fully paperless?
12) Is it easy to use?
Any vendor that can align with all of the answers you want to hear deserves a place in your 'best CAPA software' shortlist.
Now, to help you choose a suitable vendor, try our list of the best software options for managing your nonconformances and CAPAs in 2024.
Learn the 5 best options for nonconformance management software in 2024
When companies are first establishing their quality management system, CAPA software can feel like an afterthought.
After all, you probably have limited quality issues to correct or prevent in your early days. But a proactive approach, recognising and foreseeing the need for a robust CAPA software system in the future, is the best way to lay the groundwork early and ensure you invest in a suitable tool for the long term.
Make a start by booking your Qualio demo and getting your questions answered!