FDA 21 CFR Part 11 checklist PDF
Meet every requirement of FDA 21 CFR Part 11.
Download your FDA 21 CFR Part 11 checklist PDF to:
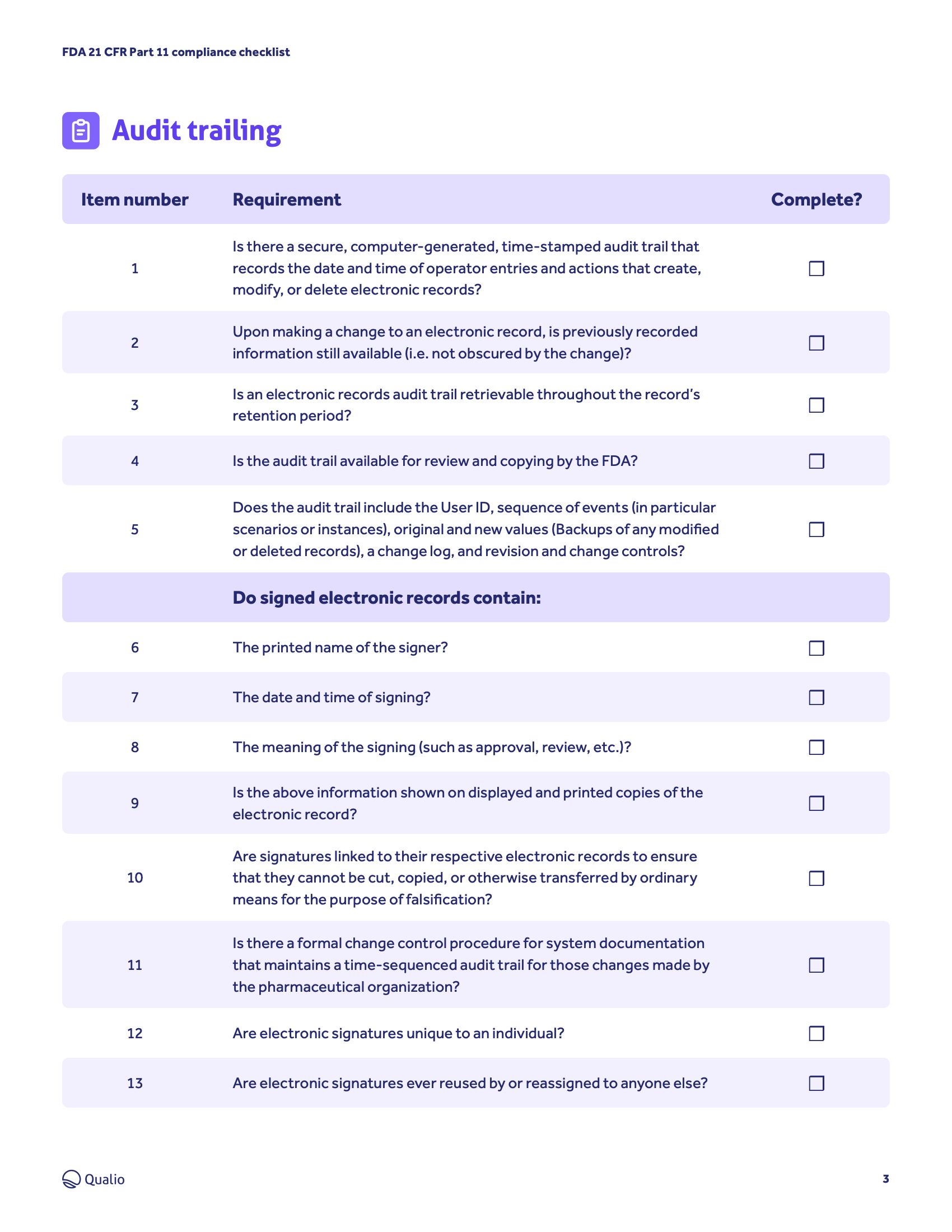
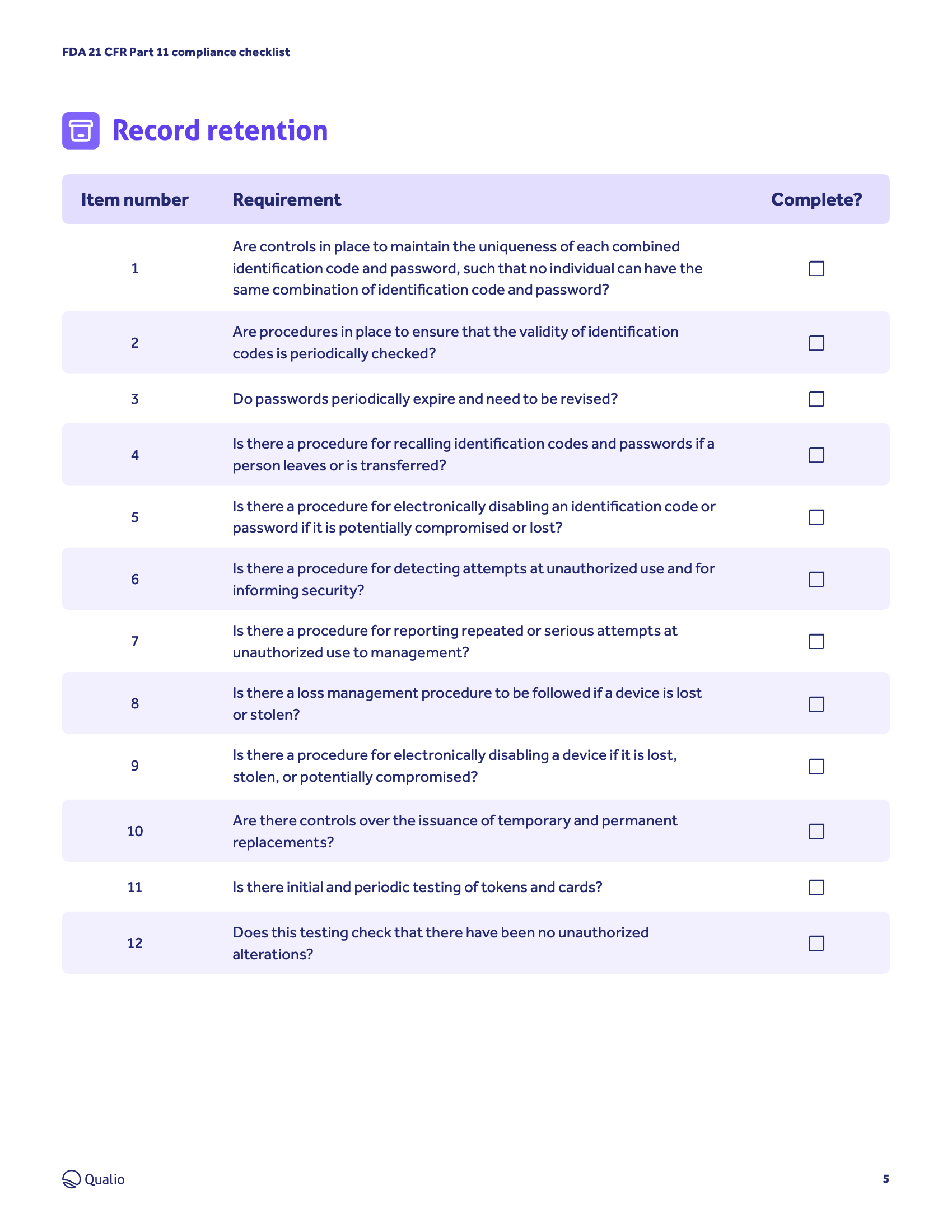
- Get to grips with the FDA's requirements for the integrity, quality and compliance of electronic records and signatures
- Understand the steps your business needs to take, from validation and audit trailing to record copying and retention
- Access an ordered step-by-step checklist and drive an organized, controlled pathway to compliance
Complete the form to the right to get started!
What you'll get:
Compliance checklist
Section-by-section blueprint
Work through a sectioned series of actions and requirements to embed compliance
Peace of mind
Tick off each requirement as you fulfil it and work towards airtight electronic record and signature compliance