Regulation isn't slowing your medical device. Fragmentation is.
For years, the medical device industry has assumed that slow product development is simply the cost of regulation.
FDA oversight is rigorous.
ISO 13485 documentation is detailed.
EU MDR technical files are extensive.
Delays are often treated as inevitable.
But in today’s medtech organizations, that assumption deserves scrutiny.
Increasingly, the primary cause of delay is not the FDA.
It's fragmented quality and compliance architecture.
The familiar pre-audit pattern
Consider the week before:
- An FDA inspection under the QMSR
- An ISO 13485 audit
- An EU MDR submission
In many growth-stage medical device companies:
- Conference rooms become command centers
- Engineering slows or pauses
- Regulatory affairs and quality assurance shift into documentation mode
- CAPA records are reconciled
- ISO 14971 risk files are reviewed
- Design controls are cross-checked
- Verification protocols are validated against the Design History File (DHF)
During this time, teams are not improving the device.
They're excavating evidence.
The search for the 'golden thread'
Medical device compliance depends on lifecycle traceability.
Regulators expect a defensible connection from:
- User need
- To design input
- To design
- To verification and validation
- To risk mitigation
- To post-market surveillance
![[DRAFT] Everything You Need to Know About Design Controls for Medical Devices](https://www.qualio.com/hs-fs/hubfs/%5BDRAFT%5D%20Everything%20You%20Need%20to%20Know%20About%20Design%20Controls%20for%20Medical%20Devices.jpg?width=1000&height=550&name=%5BDRAFT%5D%20Everything%20You%20Need%20to%20Know%20About%20Design%20Controls%20for%20Medical%20Devices.jpg)
This 'golden thread' must be visible within the quality management system (QMS) and defensible during audits and regulatory submissions.
But in many medtech organizations, the thread is scattered:
- Design inputs in PLM
- Software tickets in engineering systems
- Risk matrices in ISO 14971 spreadsheets
- Complaint records in separate databases
- CAPA workflows in a legacy QMS
- Training documentation in HR systems
Each system works independently.
Regulatory compliance evaluates the connections between them.
Where regulatory velocity slows
When an FDA investigator asks:
“How did this field complaint lead to a risk reassessment, a design update, and verification testing?”
The answer must be immediate and traceable.
If that traceability requires manual compilation across disconnected tools, regulatory velocity slows.
This fragmentation leads to:
- Extended cross-checks for regulatory submissions
- Redundant documentation within EU MDR technical files
- Complex change control analysis
- Manual impact assessments
- Audit preparation cycles that consume engineering time
This is often dismissed as administrative overhead.
It isn't. It's a structural constraint.
Why fragmentation now impacts valuation
For commercial-stage medical devices:
- Regulatory delays defer revenue
- Distribution agreements stall
- Market access timing shifts
For venture-backed medtech companies:
- FDA inspection readiness variability affects investor confidence
- CE marking unpredictability introduces funding risk
Fragmentation increases variability. And variability increases strategic risk.
Modern devices have outgrown legacy compliance models
Medical devices are no longer static hardware products.
They include:
- Embedded firmware
- Cloud-connected software
- Mobile applications
- Cybersecurity controls
- AI-driven decision support
Under:
- IEC 62304
- FDA software as a medical device (SaMD) guidance
- ISO 14971
- The EU MDR
Design controls must support continuous iteration.
Risk management must reflect algorithmic and cybersecurity risk.
Post-market surveillance must integrate real-world data.
Yet many compliance environments remain document-centric, rather than lifecycle-centric.
Static documentation cannot represent dynamic product ecosystems.
A legacy QMS may store procedures effectively.
It does not inherently:
- Link software updates to risk reassessments
- Connect risk updates to verification protocols
- Tie verification outcomes to regulatory submission artifacts
That architecture was built for a previous era.
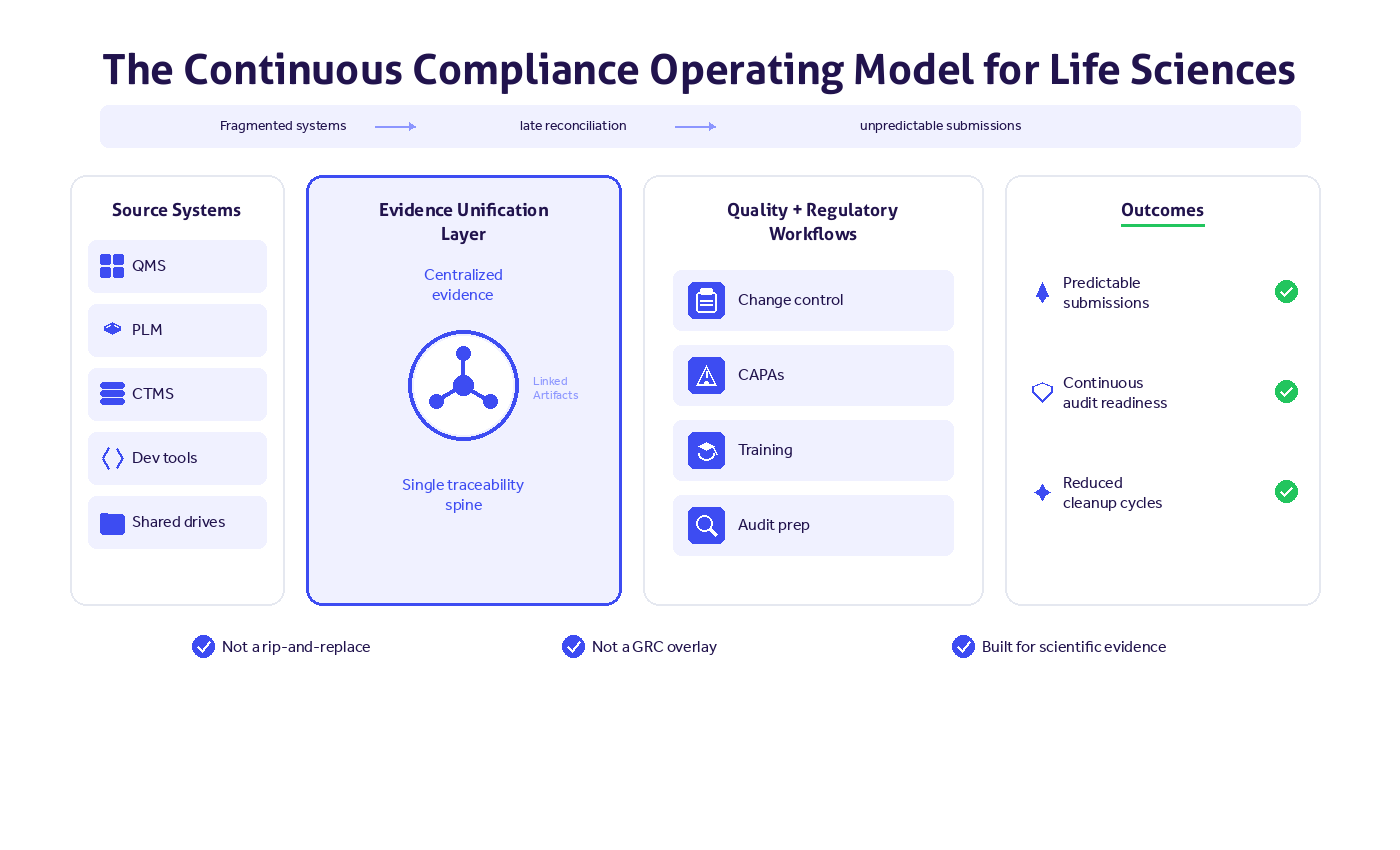
Continuous compliance as architectural strategy
Leading medtech organizations are responding with continuous compliance.

Continuous compliance means:
- Capturing regulatory evidence at the moment it's created
- Embedding lifecycle traceability into design controls
- Integrating risk management, CAPA, complaint handling, and post-market surveillance
- Creating a unified quality and compliance architecture
In this model:
- Design changes trigger structured risk reviews
- Risk updates link directly to new verification requirements
- Post-market signals integrate into the DHF
- Audit readiness is visible to leadership
Traceability is built in. Not reconstructed.
The strategic reframe
Medical device companies are not inherently slowed by:
- The FDA QMSR
- ISO 13485
- ISO 14971
- EU MDR
These frameworks exist to protect patient safety and device efficacy.
The true constraint is fragmented evidence across systems that do not support lifecycle traceability.
When compliance architecture does not reflect product complexity, regulatory speed declines.
Not because standards are too strict. But because the foundation is misaligned.
The executive question
Regulatory speed in medtech is no longer determined solely by documentation discipline.
It depends on whether your compliance foundation was built for:
- Modern software-driven devices
- Integrated risk management
- Continuous audit readiness
- Unified lifecycle traceability
As global regulatory expectations increase and device complexity accelerates, organizations must ask:
Is our quality and compliance architecture designed for today’s medical device lifecycle?
Or for a previous era?
