510(k) exempt medical devices: how to tell if you need to submit
Determining if your medical device is 510(k) exempt or not is a crucial first step as you begin to chart your regulatory pathway to market.
Most medical devices marketed in the United States do not require the completion of a 510(k) submission. But there are still thousands of devices which do pass through the process each year - and early, proactive planning is crucial for success.
Unsure if your device is 510(k) exempt? Get your answer here.
Is my medical device 510(k) exempt?
To understand whether your device is 510(k) exempt or not, it's important to first understand the risk classes which all American medical devices are bucketed into.
They look like this:
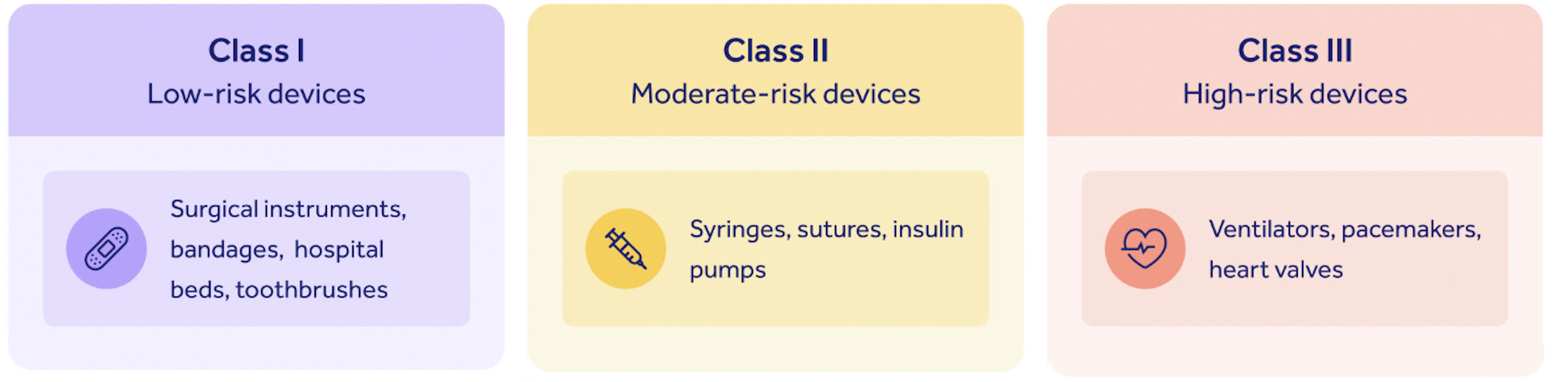
The higher the risk your device poses to the safety of a patient, the higher the risk class that your device sits in.
As a general rule of thumb, 510(k) submissions apply to Class II medical devices. This is your first clue to whether your device is 510(k) exempt or not.
Class I devices
If your device is a low-risk Class I device, as 47% of US medical devices are, you're probably 510(k) exempt.
This is because your device, according to the FDA, doesn't pose 'a potential unreasonable risk of illness or injury', and doesn't require the full regulatory screening process of a 510(k) submission.
Class I device examples include:
- Toothbrushes
- Hospital beds
- Oxygen masks
- Bandages
- Surgical instruments
NOTE: You'll notice that we used the words 'probably exempt' when referring to Class I devices. That's because there are a subset of 'reserved' Class I devices which do require a 510(k) submission and are not 510(k) exempt.
There may be other rare occasions, too, where a significant technological or intended use change to your Class I device might make a 510(k) submission necessary.
In these cases, use your best judgment and turn to a medical device consultant for help if required.
As a general rule, though, you can be fairly confident that your Class I device is 510(k) exempt.
Your device is Class I: 510(k) exempt!
Class III devices
If your device is among the 10% of medical devices falling into the high-risk Class III category, you're also exempt from the 510(k) pathway, since you'll need to follow the more rigorous Premarket Approval (PMA) submission journey instead.
Class III device examples include:
- Pacemakers
- Defibrillators
- Ventilators
- Breast implants
- Heart valves
- Cochlear implants
- Implanted prosthetics
The logic here is that these high-risk devices demand more regulatory scrutiny than a 510(k) process, which mainly centers around proving your device is substantially equivalent to another.
Just like the Class I category there's a caveat here, and a rare set of circumstances where a 510(k) submission could be appropriate for a Class III device.
That is: if your Class III device is substantially equivalent to a 'preamendment' Class III device (i.e. one marketed before the Medical Device Regulation Act of 1976, when PMAs weren't required), then you could theoretically proceed with a 510(k) submission linking your device to that preamendment predicate.
This, however, is an increasingly rare route. The FDA frowns on the use of predicates much older than a decade - so as we approach half a century since the Act, we can expect the use of pre-PMA Class III 510(k) predicates to fizzle out.
As such, here too we can be generally confident that Class III status equals 510(k) exemption.
Your device is Class III: 510(k) exempt!
To learn more about the FDA's device risk classes and their respective regulatory pathways, try our classification guide:
Other 510(k) exempt medical devices
Exempted Class II devices
There's a little more to just risk classes, though, as you try to determine if your device is 510(k) exempt or not.
For instance, not every single Class II device requires a 510(k) submission by default. The FDA's Product Classification database contains a list of over 800 devices that are 510(k) exempt, according to the 1997 FDAMA or the 2016 Cures Act.
These Class II devices are considered to be 'low-medium risk', and the FDA's stance on exempting them from the 510(k) process is to allow the regulator to 'redirect resources' to 'more significant public health issues'.
To find out if your Class II device is 510(k) exempt:
1) Visit the Exemptions list
2) Click into the one of the 18 'specialties' that your device falls under
3) Scan for devices marked '(II)' - these are Class II devices
4) See if your device is on the list
Your Class II device is on the list: 510(k) exempt!
It's important to note here that even if your device is exempt from the 510(k) process, you'll still need to follow the cGMP requirements expected of a Class II device.
Class II devices without a predicate
The 510(k) process hinges on proving that your device is safe and effective by pointing to an existing 'predicate' device with similar characteristics that's already on the market.
It's possible that your business has a Class II medical device that's particularly novel and innovative. While it's a good thing that you've found a market niche, it also means you may struggle to find a suitable predicate device.
If you've done your search and really can't find one, you're exempt from the traditional 510(k) process and must instead follow the de novo route.
Your Class II device has no predicate: 510(k) exempt!
Medical device data systems (MDDS)
In September 2022, the FDA's "Medical Device Data Systems, Medical Image Storage Devices, and Medical Image Communications Devices" guidelines were updated from their original 2015 state to revise how 'medical device data systems' (MDDS) and other digital medical platforms are regulated.
In short, the crux of the matter is that the definition of a medical device has been tightened: MDDS are now no longer classed as medical devices in the regulatory sense of the term, and don't therefore require the same regulatory pathways, such as a 510(k) submission.
Your MDDS - whether a hardware 'device-MDDS' or a software 'non-device-MDDS' - will therefore be 510(k) exempt under the new FDA guidelines.
Dive deeper into the 2022 MDDS guidelines with our analysis blog post
As usual, though, there are a few exceptions for some MDDS which provide a more critical data function, such as:
- Specialized medical display hardware devices for mammography, radiology, pathology, and ophthalmology
- Other medical display hardware integral to the safe and effective use of a medical device hardware product, such as 3D displays for robotic surgery systems or digital displays within ICU bedside monitors
If your MDDS sits in these categories, and meets any of the other criteria we've discussed here, then it won't be 510(k) exempt.
For the vast majority of MDDS, though, we can summarize by saying:
Your device is an MDDS: 510(k) exempt!
Which medical devices are 510(k) exempt?
Taking it all together in conclusion then, your device is 510(k) exempt if:
1. It's Class I, and not 'reserved'
2. It's Class III, and you can't get away with a preamendment predicate
3. It's Class II, and on the exempt list
4. It's Class II, and no suitable predicate exists
5. It's an MDDS - and therefore, now not classed as a device at all!
Other regulatory requirements will of course still apply, and you shouldn't let your guard down and ignore them.
But falling into any of these categories means your medical device is 510(k) exempt and won't need to go through the process of proving substantial equivalence to a predicate.
