Medical device classification PDF guide
Pinpoint your medical device class and your steps to market.
Download our medical device classification PDF guide to:
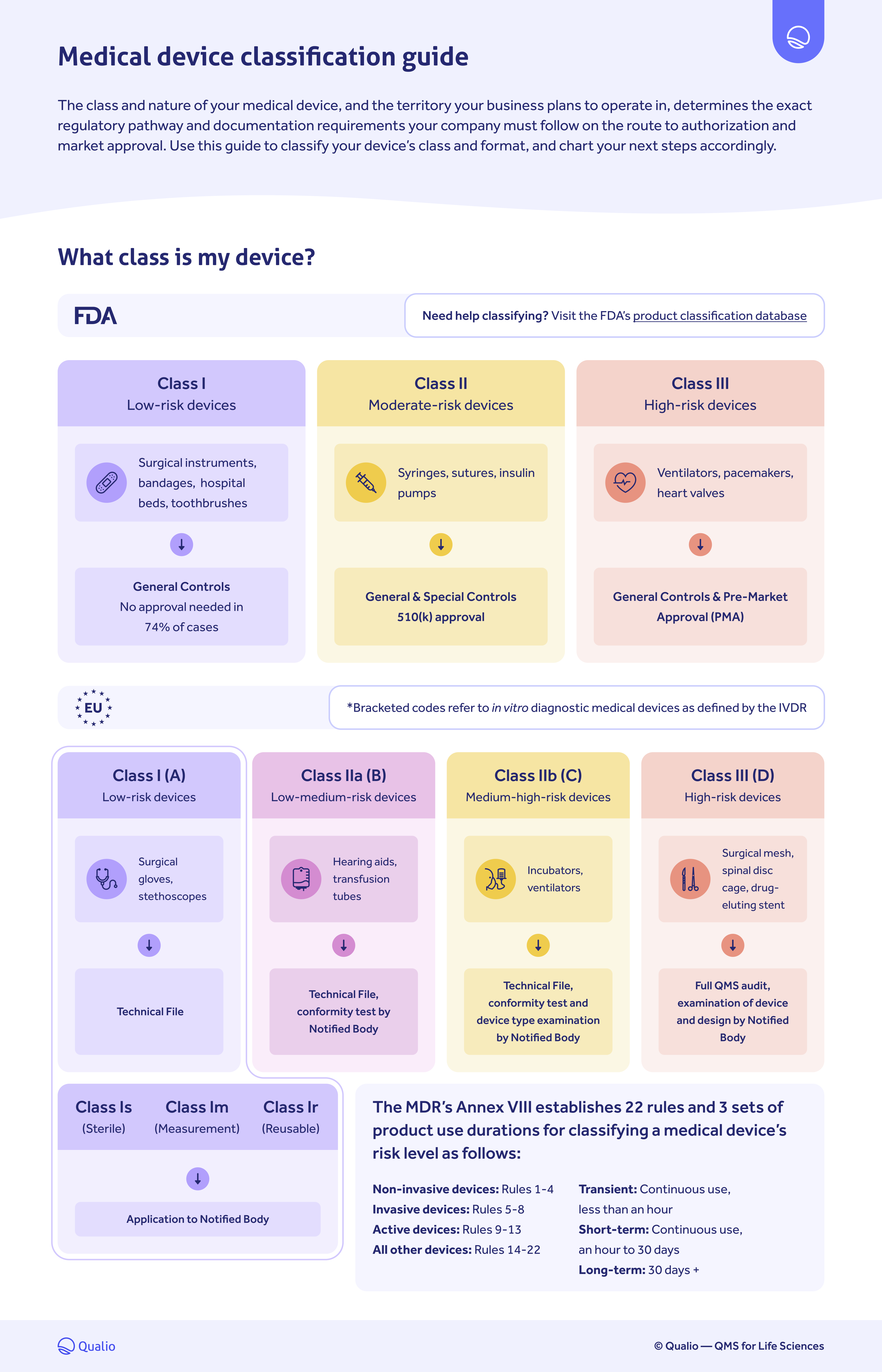
- Understand what regulatory class your medical device falls under for FDA and EU compliance
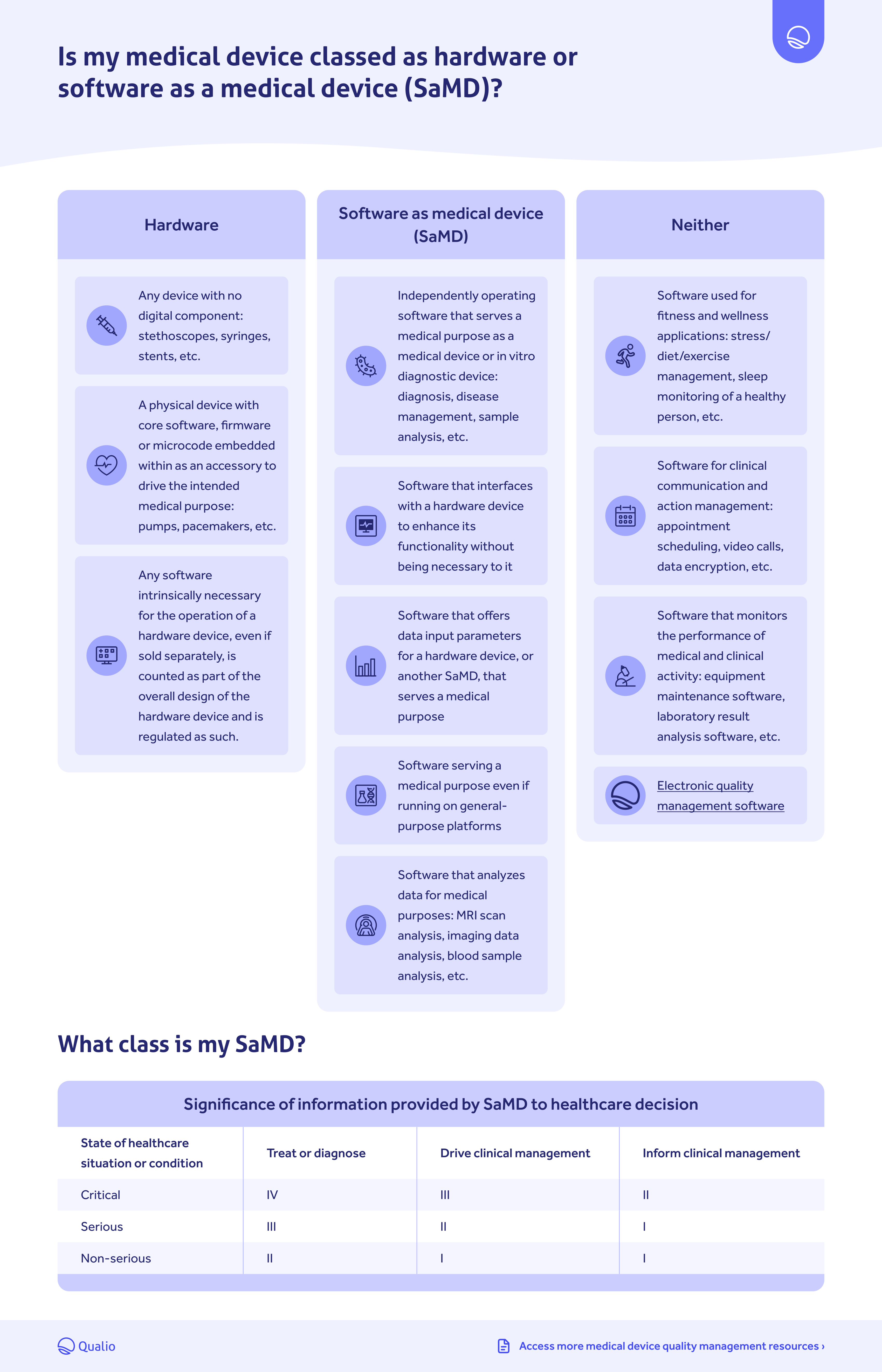
- Determine whether your medical device classes as a hardware or SaMD product
- Chart your regulatory pathway accordingly
Complete the form to the right to get started!
What you'll get:
FDA and EU medical device classification breakdown
Hardware vs. SaMD comparison
Regulatory pathway clarity