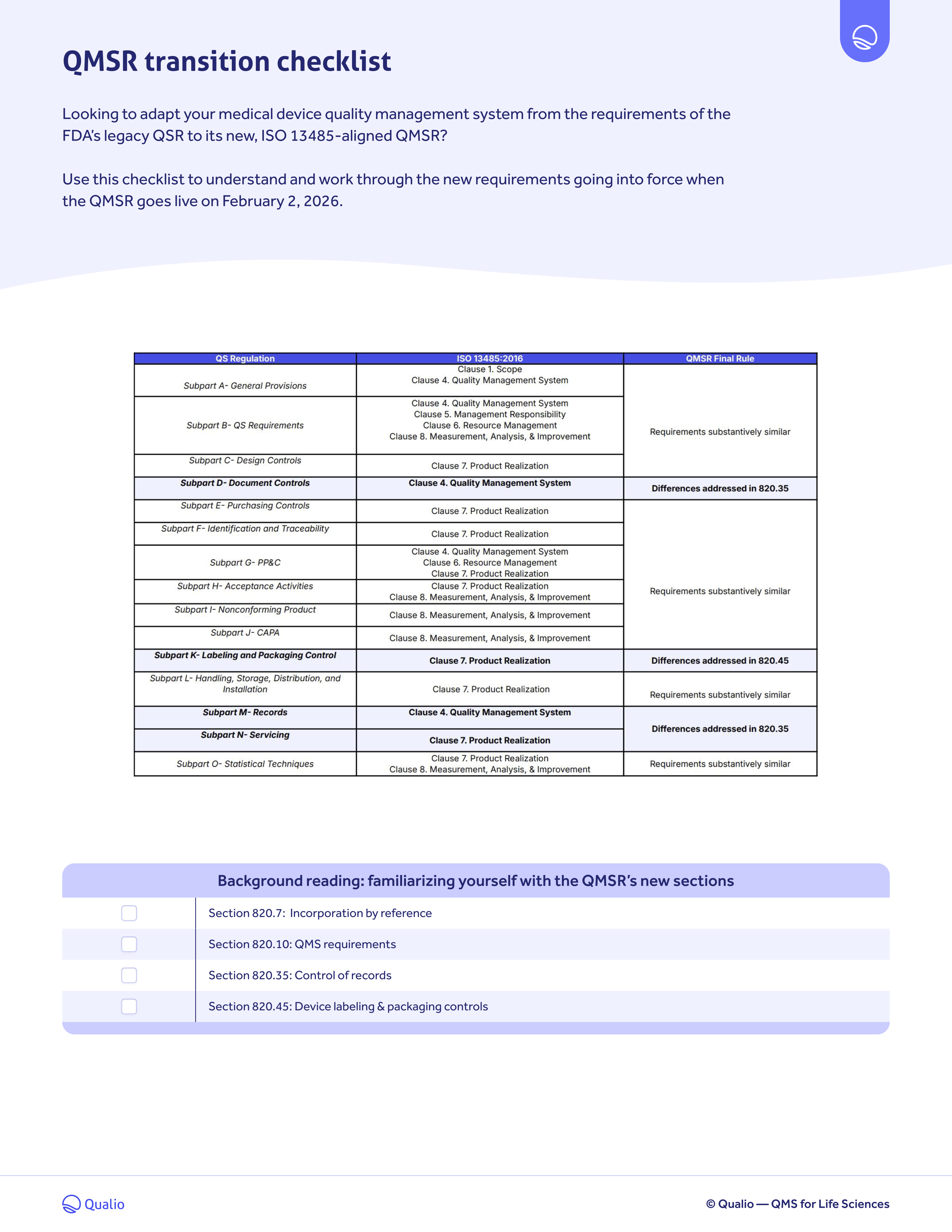
QMSR transition checklist
Make a smooth, controlled transition to the FDA's new QMSR requirements.
Download your QMSR transition checklist PDF to:
- Understand where the FDA's new QMSR requirements differ from its legacy QSR
- Know which requirements, from ISO 13485 to Part 820, prevail in the FDA's new harmonized regulation
- Work through each clause and check off your compliance ingredients one by one
What you'll get:
QMSR transition checklist
Section-by-section blueprint
Work through a sectioned series of medical device QMS requirements to get everything you need in place for FDA compliance
FDA audit confidence
Work through each requirement to gain airtight FDA inspection readiness and full confidence you're in compliance with the Final Rule