
Restech produces a suite of medical devices for minimally invasive diagnosis and treatment of reflux disorders.
The company's impressive growth and its portfolio of devices has demanded a carefully constructed QMS to support operations in multiple international territories.
Alongside FDA 21 CFR 820 and ISO 13485 compliance, Restech is transitioning from the EU's MDD to its MDR, with a longer-term goal of UK MHRA approval too.
Restech and its Director of Quality Assurance Lowell Hoffman ran a manual, paper-based quality management system.
Lowell knew strong document control was at the heart of the QMS, but their old ways of working involved drawn-out, labor-intensive document management processes.
The amount of time and resources needed to maintain our old QMS was tremendous.
The hours lost trying to keep up with the paperwork were less than an eQMS system payment.
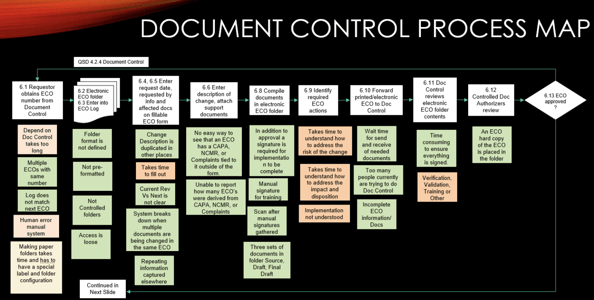
Restech's document management was a manual headache
The adoption of an electronic quality management system (eQMS) to eliminate admin time and lift the lid on quality-centric growth had been a long-term Restech priority for several years.
Well aware of the powerful business benefits offered by digital quality, Restech's senior management team tasked Lowell with sourcing the best medical device eQMS platform.
Since the systems Lowell found all offered broadly similar functionality, the deciding criteria had to be:
Cost
Ease of use
Look and feel
Ease of set-up and transfer from the old QMS
Long-term customer support
Taking all these factors into account, Lowell pinpointed Qualio as the optimal choice, and Restech became Qualio customers in 2022.
Replacing paper with cloud-powered digital quality had an instant impact.
Lowell and other Restech department leads could hand off their full-time QMS upkeep tasks to a single Qualio administrator, freeing them to focus on value-add activities.
Fast and easy digital training boosted internal training completion to 100%, allowing Lowell to achieve a perfect ISO 13485 audit with 0 non-conformances.
And Lowell and the quality team can now focus on long-term company initiatives, confident that core QMS actions like document control and compliance are taken care of in the background.
Buoyed by the dramatic success of their Qualio investment, Restech now plan to roll the software out to their San Diego facility, expanding system usage to prepare them for compliant operation in the lucrative European and British medical device markets.
It wasn't just a sales pitch with Qualio. They told us what was going to happen and they executed exactly.
When I didn't know how to do something, Casey made a video to show me.
To me, that's where the value is. We're a partner, and Qualio treats us like partners.
That's probably the rarest of all when you're dealing with software companies in this area.
