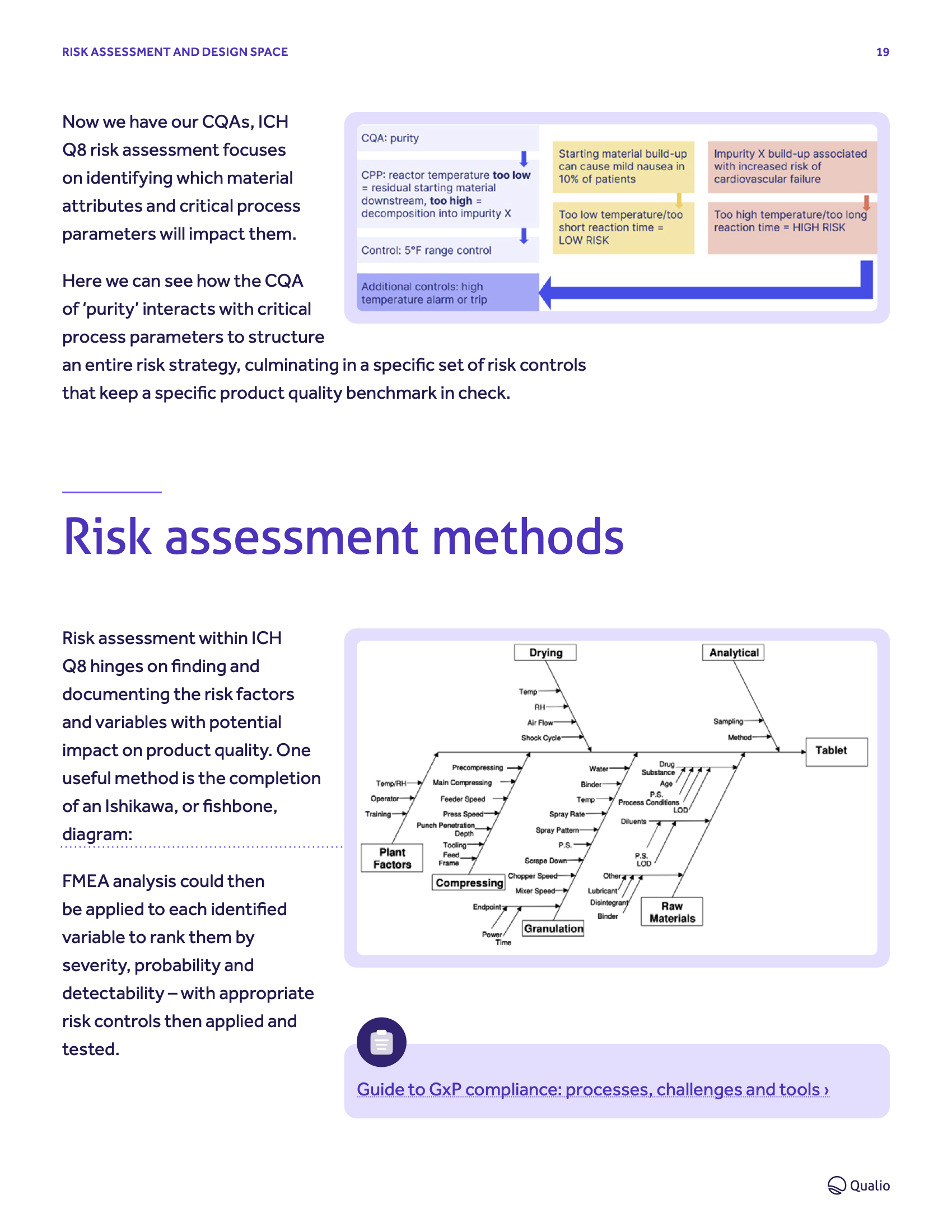
How to prepare for ICH Q8 compliance PDF guide
Embrace quality by design for your pharma or biotech business.
Learn everything you need to get ICH Q8 best practice in place.
Download our ICH Q8 guide to:
- Understand the core requirements of ICH Q8, the pharmaceutical development standard
- Embed business-wide ICH Q8-compliant quality by design (QbD)
- Lift your pharma manufacturing to new levels by mastering quality ingredients like CQAs and QTPPs
What you'll get:
ICH Q8 compliance guide
Understand what ICH Q8 is and what you need to do for compliance
Quality-led pharmaceutical manufacturing
From building a design space to implementing a quality-by-design control strategy, learn how to put quality front and center of your manufacturing line
Roadmap for the future
Regulators are placing unprecedented emphasis on continuously improving quality for pharmaceutical and biotech businesses. Keep your business competitive and fighting-fit by embracing the latest best practice