Complete guide to the FDA Quality Management Maturity program
The FDA's QMM program promises a radical shake-up of how pharmaceutical quality is perceived, measured and rewarded.
Learn how to align with the program and use it to your advantage with our comprehensive guide.
Download our Quality Management Maturity program guide to:
- Understand the FDA's plans for its innovative new pharmaceutical quality program
- Get to grips with the program's expectations and how to align with them
- Learn how to optimize your own quality maturity and unlock the full rewards of the program
What you'll get:
QMM program breakdown
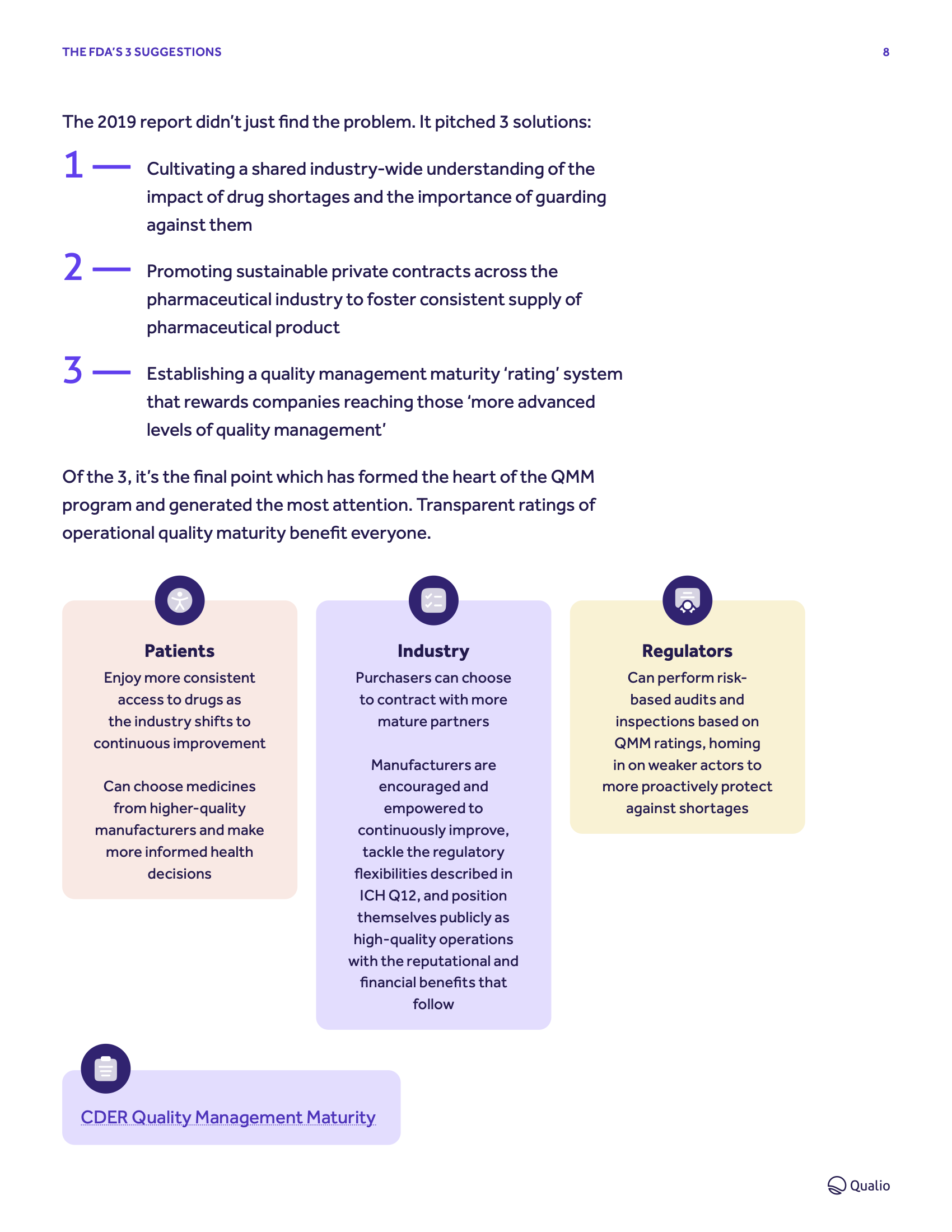
Explore how the FDA's Quality Management Maturity program will impact the pharmaceutical industry and kickstart a new approach to industry quality
Mature PQS roadmap
What does 'quality management maturity' mean in reality? How can you embed it - and then prove it? Get proactive and hit the program's expectations early for maximum benefits
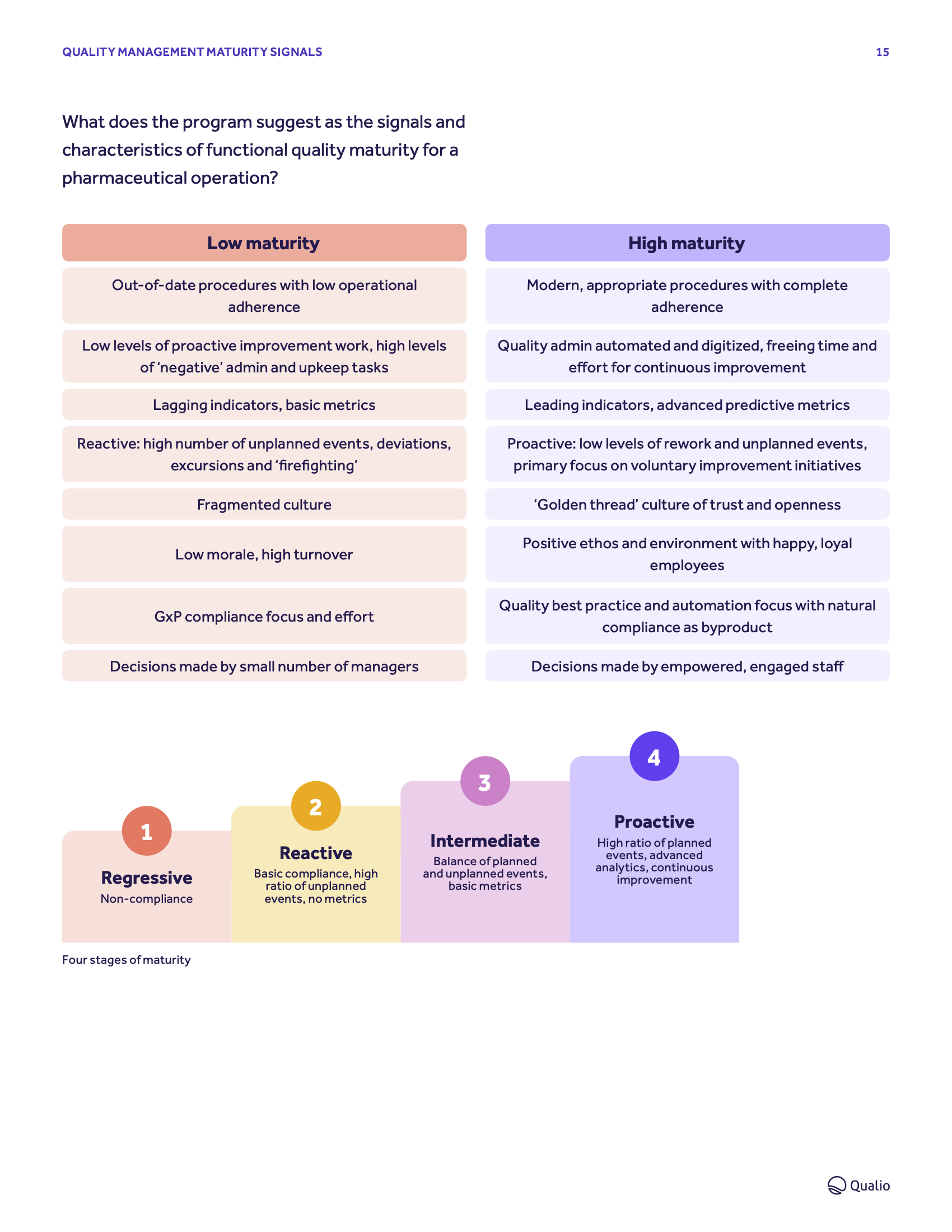
Signals and ingredients
Learn how to identify, measure and lift your own maturity levels to usher in optimized, world-class quality