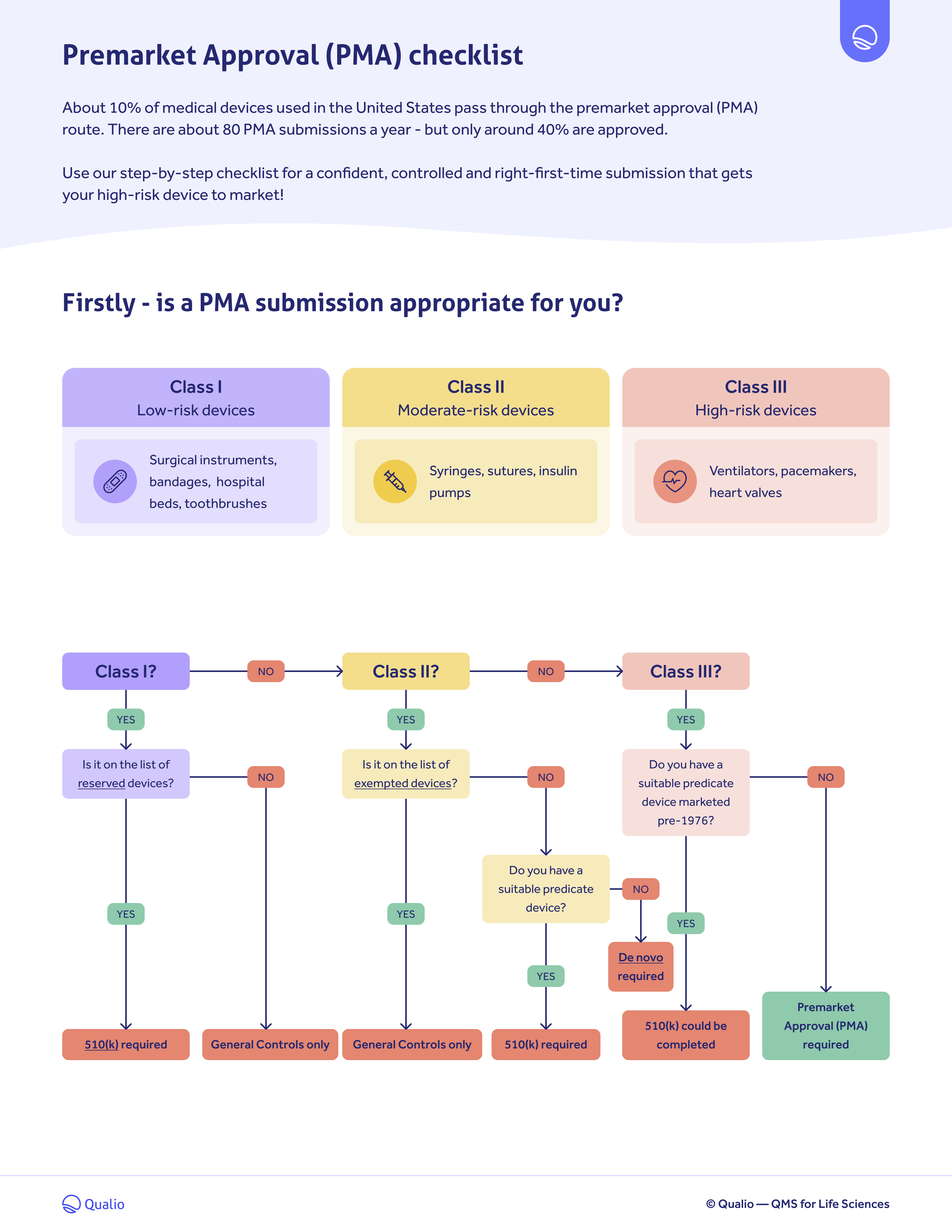
FDA PMA submission checklist
Work through each FDA PMA submission requirement and get your Class III device to market with our checklist.
Instant access. No email required.
Download our FDA PMA submission checklist to:
- Work through the key steps and requirements of the FDA premarket approval (PMA) submission process
- Ensure nothing is missed from your medical device QMS and your submission preparations
- Get your Class III medical device to the US market in a controlled, step-by-step process
What you'll get:
Step-by-step guidance
Work through the PMA process, from building a Class III medical device QMS to assembling your submission ingredients
Submission confidence
Know what you need to do for a successful FDA approval
Class III device FDA sign-off
Drive a PMA process that gets your high-risk device approved for US market entry