Contract organization quality management software datasheet
See why CROs and CMOs across the world use Qualio.
Download our contract organization quality software datasheet to learn how Qualio:
- Gives contract research and manufacturing organizations a trusted and compliant quality management framework
- Supports and enables the CMO cGMP requirements of FDA 21 CFR Part 210/211 and the CRO requirements of ICH E6 (R2) and ISO 14155
- Brings your quality management activity together into a harmonized, controlled and sponsor-trusted digital repository
Complete the form to the right to get started!
What you'll get:
Feature breakdown
Explore the core features and functionality of Qualio, and how each area supports world class CxO activity
Happier sponsors
Learn how Qualio mitigates risk, embeds unshakeable quality control and lets you build stronger, happier relationships with your sponsors
Holistic and quality-centric contract research/manufacturing approach
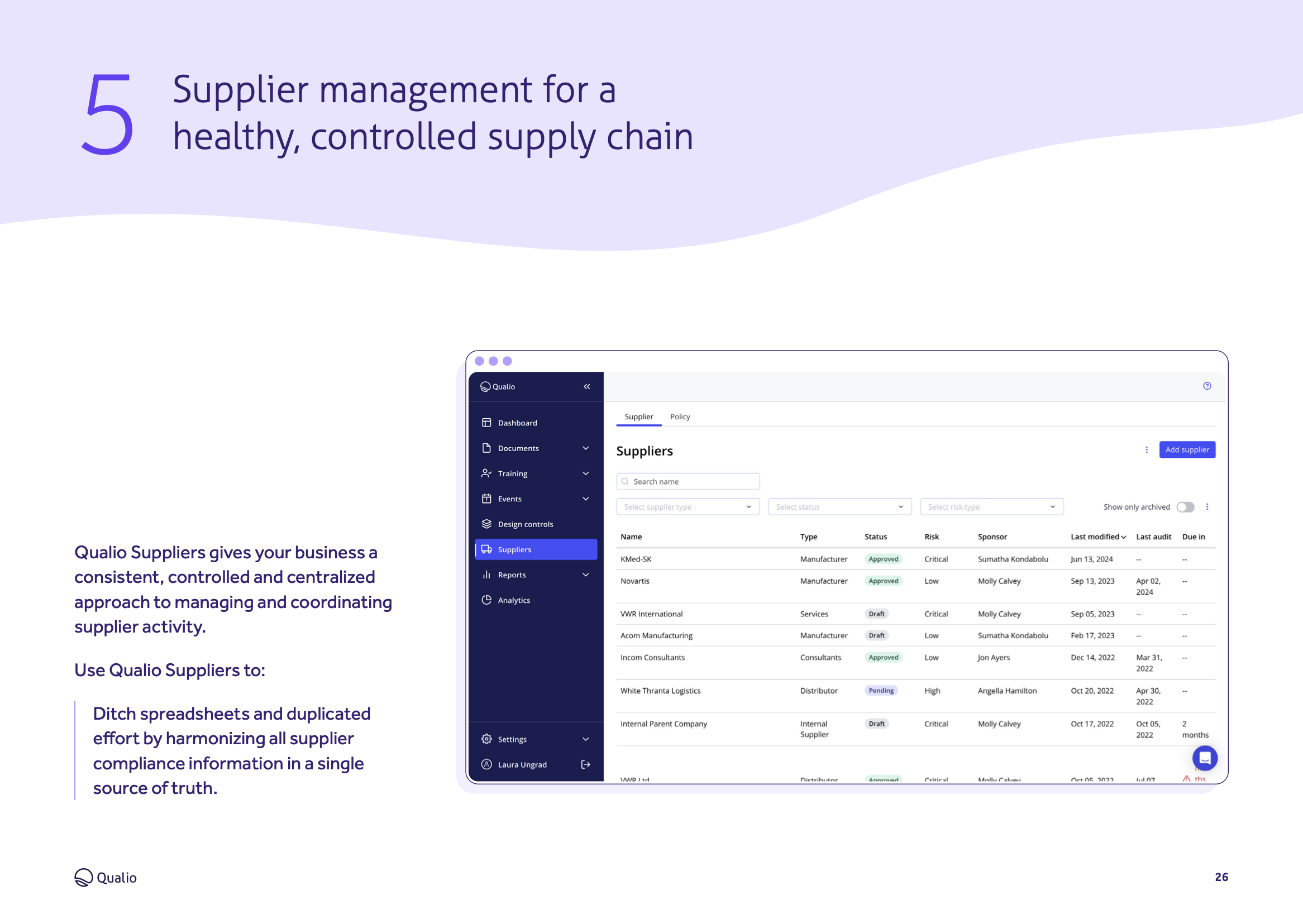
Understand how to manage documents, training, suppliers, analytics, quality events and design controls in a single easy-to-use system that automates and guarantees compliance