Pharma company achieves 5x ROI with Compliance Intelligence
LogixX Pharma uses Compliance Intelligence to slice consultant spend and accelerate ISO 15189 readiness by 80%
"For what you spend on Compliance Intelligence, you get it back at least fivefold.

Michael Close
CEO, LogixX Pharma
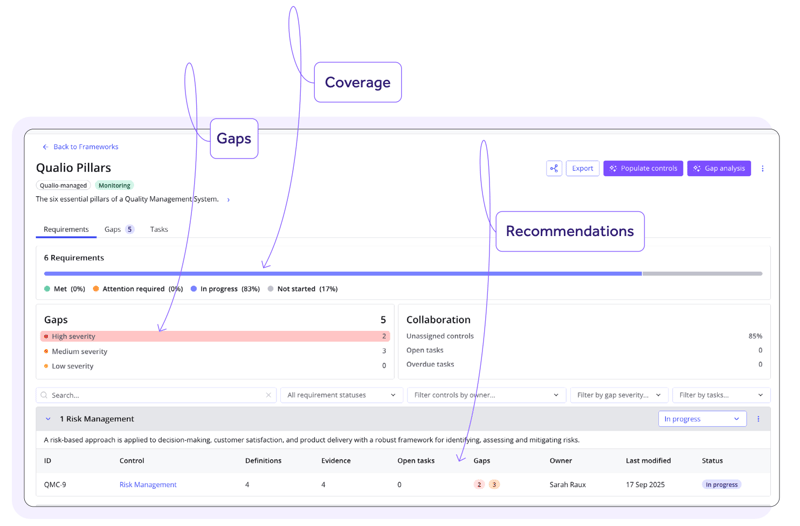
LogixX Pharma's story
The results
Immediate return on investment
Automated compliance processes sliced consultant spend and accelerated the company's strategic plans by 80%, delivering clear and rapid ROI in the first 2 months.
| Activity | Before Compliance Intelligence | After Compliance Intelligence |
|---|---|---|
| Audit prep | Expensive consultant support requiring ramp time and manual work | Instantly available, 30-40-minute automated scan of entire QMS: 60% of consultant spend mitigated |
| ISO 15189 accreditation prep | Months of manual gap analysis and document/process-building | 80% reduction unlocked by AI gap analysis and step-by-step compliance guidance |
Compliance Intelligence guided LogixX Pharma through a successful ISO 13485 audit, then set the company up to tackle their ISO 15189 accreditation target at speed.
"The tool's saved us masses and masses of time. The value is definitely there.
Thinking about Compliance Intelligence? Go for it.
How it works
| 1. Scan | Entire QMS scanned for gaps against your regulatory standards (ISO 13485, ISO 15189, etc.) in 30–40 minutes, ending reliance on consultants |
| 2. Prioritize | Compliance Intelligence organizes gaps by severity and significance to guide resource allocation and accelerate prep time by 80% |
| 3. Fix | Auto-create tasks with owners and timelines to close gaps and get audit-ready at speed |
| 4. Verify |
Re-scan to confirm fixes and compliance |
| 5. Audit readiness forever |
Continuous monitoring and flagging ensures gaps are always spotted and fixed before your auditor ever sees them |
How can Compliance Intelligence deliver 5x ROI?
Bringing compliance expertise in-house with Compliance Intelligence, like Michael and the LogixX Pharma team did, lets your company easily prepare for audits without expensive ongoing or bespoke external consultancy support, saving significant time and effort from external QA reviewers.
The LogixX Pharma team estimates that Compliance Intelligence has saved them at least 1 FTE external QA expert, and months of time conducting gap analysis of their QMS system. Michael also pointed out that the external consultant gap analysis would only be as good as the training or knowledge that the external expert had received – which may not be relevant to your business. Compliance Intelligence removes compliance ambiguity with applied AI remedies.
Cutting weeks and months of manual work accelerates your company's strategic objectives, too, bringing ISO standard readiness forward by 80% and getting you ready to expand into new markets at speed.
And Compliance Intelligence ensures you don't only prepare for your audits faster, but pass them with confidence — and without regulatory fines, recalls or reputational damage.
Shielding your company from regulatory punishment is one of the best things you can do for your bottom line when you consider the average costs of compliance slip-ups in, for example, the United States:
- Cost of a Form 483 observation: $100-200K
- Cost of a warning letter: $50-250M
- Cost of an import alert: $150-500M
- Cost of a consent decree: $2B
How does Compliance Intelligence know what to assess our QMS for?
The gap assessments and remediation recommendations provided by Compliance Intelligence are based on the expert-built, pre-validated frameworks that you have turned on within the tool.
Frameworks are the clause-by-clause requirement maps of the standards and regulations that matter to you.
From FDA 21 CFR Part 11 to ICH Q10, the QMSR and ISO 13485, just turn on a framework and Compliance Intelligence assesses your level of compliance with its requirements — so you can see at a glance which you’ve met, and what you
still need to do.
We already pass all of our important audits for the year and our QMS is in good shape. What would I need Compliance Intelligence for?
Audit preparation is only one of the many ways CI helps accelerate your compliance and product milestones!
Continuous compliance is a system of intelligence built not only to help you with point-in-time compliance events like audit preparation, but to help your organization be constantly compliance-ready. It does this by automating gap analysis and remediation, and proactively alerting you about compliance issues — and suggested resolution tasks.
Here are the use cases that Compliance Intelligence is designed to address:
- Built-in compliance: pre-built frameworks and controls
- Automated gap analysis: AI-powered assessment in minutes vs. weeks
- Remediation management: Systematic management of compliance risks
- Continuous observability: Real-time monitoring of compliance with total visibility for leadership
About LogixX Pharma
Headquarters
—Berkshire, UK
Size
—11-50
Industry
—Pharmaceuticals, nutraceuticals, medical devices
UK-based LogixX Pharma specializes in the commercialization of life science products, marketing and distributing a range of innovative pharmaceuticals, nutraceuticals and medical devices.
The company has two main divisions: one handling distribution of drugs for pharmaceutical manufacturers, and one offering DNA fragmentation diagnostics for fertility clinics.
When LogixX Pharma began their Compliance Intelligence usage in 2025, the company was maintaining ISO 13485 compliance and preparing for a recertification audit, as well as investing in a new London laboratory — making ISO 15189 accreditation a top priority.

SEE COMPLIANCE INTELLIGENCE FOR YOURSELF
Get audit-ready in weeks.
Stay audit-ready forever.

