CRO unlocks a new compliance approach with Compliance Intelligence
How a Canadian CRO uses Compliance Intelligence to scan its entire QMS in 30–40 minutes and walk into sponsor audits calm and ready
"CI cuts the stress. We monitor everything at once and stay audit-ready.
Chris Rice
Senior Quality Assurance Manager, AGADA Biosciences
The story
The results
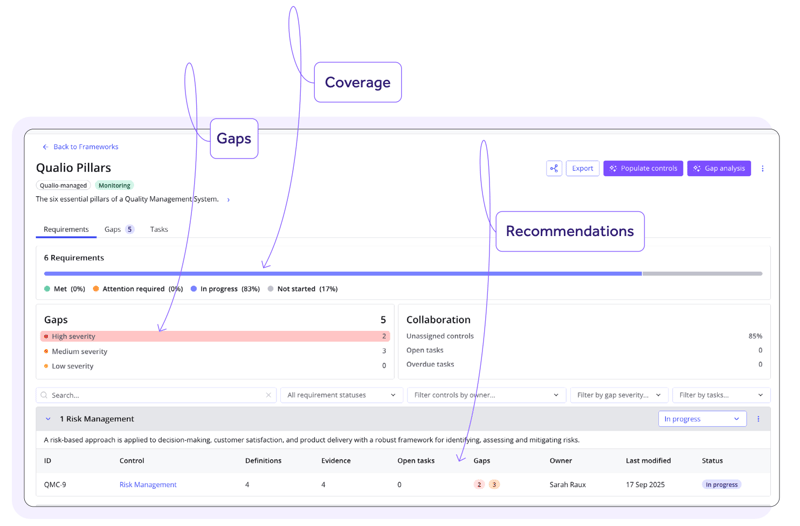
From reactive compliance to value-add activity
A weekly scramble of manual gap-finding across hundreds of records became a 30–40 minute, full-system scan by Compliance Intelligence, freeing hours each week for proactive value-add activity.
| Activity | Before Compliance Intelligence | After Compliance Intelligence |
|---|---|---|
| Finding gaps | Hours of manual QMS checks | 30-40-minute scan of entire QMS (SOPs, records, PDFs) |
| Audit prep time | 1 week | Clear reduction in prep time, freeing QA time for value-add continuous improvement |
"My confidence in being audit-ready is dramatically improved… one organized system that’s a lot easier to maintain.
How it works
-
Scan — Assess SOPs, records, and attachments in 30–40 minutes
-
Prioritize — Map gaps against the tool's built-in regulatory frameworks
-
Assign — Auto-create tasks with owners and timelines for the highest-risk items
-
Verify — Re-scan to confirm fixes; recurring issues drop off subsequent analyses
-
Stay ready — Continuous monitoring keeps you ready for sponsor and regulator audits with total confidence
How does Compliance Intelligence know what to assess our QMS for?
The gap assessments and remediation recommendations provided by Compliance Intelligence are based on the expert-built, pre-validated frameworks that you have turned on within the tool.
Frameworks are the clause-by-clause requirement maps of the standards and regulations that matter to you.
From FDA 21 CFR Part 11 to ICH Q10, the QMSR and ISO 13485, just turn on a framework and Compliance Intelligence assesses your level of compliance with its requirements — so you can see at a glance which you’ve met, and what you
still need to do.
What's the return on investment of the tool?
Cutting days of audit prep time, like AGADA did, gives your team more time to optimize processes, close gaps, and stamp out audit findings before your auditor ever spots them.
Bringing compliance expertise in-house with Compliance Intelligence also lets your company prepare for audits without consultancy support, saving, on average, $150–$300K per year.
Compliance Intelligence ensures you don't only prepare for your audit at speed, but pass it with confidence — and without regulatory fines, recalls or reputational damage.
Shielding your company from regulatory punishment is one of the best things you can do for your bottom line when you consider the average costs of compliance slip-ups:
- Cost of a Form 483 observation: $100-200K
- Cost of a warning letter: $50-250M
- Cost of an import alert: $150-500M
- Cost of a consent decree: $2B
We already pass all of our important audits for the year and our QMS is in good shape. What would I need Compliance Intelligence for?
Audit preparation is only one of the many ways CI helps accelerate your compliance and product milestones!
Continuous compliance is a system of intelligence built not only to help you with point-in-time compliance events like audit preparation, but to help your organization be constantly compliance-ready. It does this by automating gap analysis and remediation, and proactively alerting you about compliance issues — and suggested resolution tasks.
Here are the use cases that Compliance Intelligence is designed to address:
- Built-in compliance: pre-built frameworks and controls
- Automated gap analysis: AI-powered assessment in minutes vs. weeks
- Remediation management: Systematic management of compliance risks
- Continuous observability: Real-time monitoring of compliance with total visibility for leadership
About AGADA
Headquarters
—Halifax, Nova Scotia, Canada
Size
—11-50
Industry
—CRO
AGADA Biosciences provides expert assistance with drug development programs from its base in Halifax, Nova Scotia.
The company’s objective is to accelerate the route to market for drugs which tackle rare diseases, particularly neuromuscular afflictions.
AGADA offers an array of regulatory testing and research, and is a highly regulated CRO audited by both sponsors and regulators.
SEE COMPLIANCE INTELLIGENCE FOR YOURSELF
Get audit-ready in weeks.
Stay audit-ready forever.

