PMA submissions: 4 PMA application methods for medical devices
Choosing the correct Premarket Approval (PMA) method can mean the difference between the success or failure of your growing medical device startup or scale-up.
Pick the wrong PMA application method, and you’ll end up wasting valuable time, energy and money.
This wasted effort can stifle business growth overnight. In fact, recent studies indicate that 20% of life science organizations fail within the first year of business.
For some medical device start-ups, the approval process can be slow and arduous.
So what’s the best way to improve your chances of success and achieve rapid PMA submission approval?
Start by identifying the appropriate PMA application method for your unique medical device. It’s a relatively simple step that will pay significant dividends later.
To help you out, we’ve identified four popular PMA application methods for medical devices. Use this guide to quickly and easily identify the application method that your business should follow to streamline FDA approval for its new medical device.
First, let's take a look at what exactly a PMA submission is.
Introduction to PMA submissions
The PMA submission process is designed to protect the American public from the risk of defective high-risk medical devices.
Class III devices, those sustaining and supporting life or implanted within the body, are naturally treated with the highest level of risk-based caution by the FDA, and it's these devices that must pass through the PMA filing process before they can be cleared for marketization.
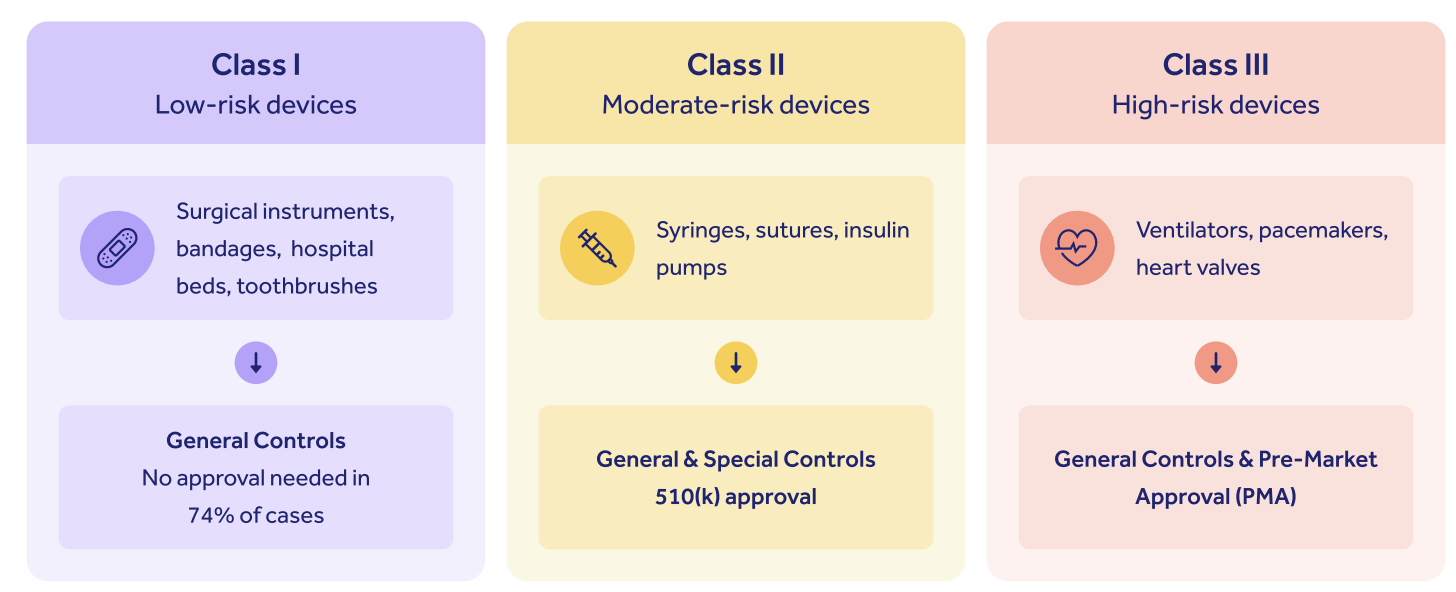
Importance of PMA for medical devices
The PMA submission process is important because it helps to guarantee the quality, safety and integrity of critical life-saving devices, ensuring patients have trust in them and are adequately treated by them.
If you're a Class III device manufacturer, you'll need to complete a PMA submission.
Because your device poses the greatest risk of patient injury or death, being able to prove that it functions properly and is underpinned by a robust medical device quality management system is of utmost importance.
It's here that the PMA submission becomes a critical hurdle on your route to the US market.
Understanding the PMA submission process
You'll need deep and confident knowledge of PMA submissions to achieve first-time success.
Let's start with the obvious: how to prepare for your PMA submission journey.
How to prepare to make a PMA filing
Begin your PMA submission journey by being absolutely sure that it applies to you.
Is your device definitely a Class III device? Visit the FDA's PMA database to check out similar devices to yours and make a decision.
Once you've determined that a PMA filing is right for your device, it's time to determine which PMA submission type is best for your operation (more on those below!)
And if you're a small business, you should complete FDA Form 3602 at this point to register as one. Small business status knocks $331K from your PMA submission fee, and is definitely worth securing.
Once you've picked your PMA filing route, it's time to get your PMA submission ingredients in order.
What is required in PMA submissions
The PMA submission, befitting the high-risk nature of your Class III device, is highly rigorous and in-depth.
Your PMA filing will require documentation including:
- Descriptions of your device, what it does, and how it works, at various levels of complexity
- Lots of technical data: both non-clinical laboratory and clinical trial data sets, with results and conclusions
- Any reports or journal entries about your device
- Financial certification or disclosure statements, depending on whether your clinical investigators have been paid for their work or not
- Samples
- Labels
FDA PMA review timeline
How long does the FDA PMA submission process take?
As you might expect, the depth and rigor of the process takes time. In fact, a PMA filing review takes about 180 days - twice as long as the 510(k) process required for medium-risk devices.
The PMA submission review comprises 4 key steps:
1) Initial review to check your PMA submission contains everything it should
2) The proper 'substantive' review, where the FDA checks your PMA submission and requests any clarification where necessary
3) A panel review if your device is particularly unique
4) The decision announcement, within 180 days of the beginning of the substantive review
What are the 4 PMA application methods for medical devices?
All Class III medical device manufacturers must secure PMA before manufacturing, marketing, and distributing products. In most cases, barring any unforeseen issues or circumstances, the FDA approval process rarely takes longer than 180 days in total.
There are four types of PMA application methods, including a traditional PMA, modular PMA, product development protocol (PDP), and humanitarian device exemption (HDE).
During the approval process, you demonstrate a commitment to product quality. Many medical device manufacturers invest in quality management system (QMS) solutions designed to improve document management, design control, training, quality reporting, and more. Unfortunately, paper-based and other traditional “systems” rarely deliver the intelligence necessary for rapid and sustained regulatory approval.
Beyond investing in a modern and advanced QMS from the outset, you can maximize your chances for FDA approval by reviewing the top four PMA application methods listed below.
1. Traditional PMA
Traditional PMA is the most common method for achieving FDA clearance. This is an ideal application method for manufacturers that have already completed clinical testing.
CFR 21 Part 814 outlines the unique requirements and documents needed to achieve rapid approval. Typically, applicants start by creating a brief summary of their devices.
As you prepare your own device summary, try to answer the following questions:
- What is the intended application of the device?
- What technical data can you provide to prove device reliability and effectiveness?
- Does the device align with major performance standards?
When submitting an application using the traditional PMA method, you must submit an electronic copy (eCopy). Before submitting your application, reference the Premarket Approval Application Filing Review to benchmark your application against the process/requirements set forth by PMA reviewers.
RELATED READING: How to Shrink the FDA Medical Device Approval Timeline
2. Modular PMA
Modular PMA is a well-designed application method for devices that have not completed clinical testing. To achieve FDA approval, applicants must complete designated “modules.” Each completed module is submitted to the FDA, and final confirmation is granted once the applicant completes all sections of the PMA.
It’s a relatively simple process that helps to create a well-designed (and compliant) medical device over time. Additionally, the PMA Shell makes it easy to understand what’s required during each phase of the application.
For a complete list of Modular PMA requirements, review the following documents: Premarket Approval Application Modular Review.
3. Product Development Protocol
Product development protocol is another PMA application method. This process is tailor-made for medical devices that utilize technology pre-approved by the FDA. Additionally, Class III devices may benefit from this approval pathway.
Applicants are required to submit “milestone reports” that outline all design and development outputs. In short, these milestone reports are used to demonstrate device effectiveness and safety. The interaction with the FDA through PDP allows applicants to quickly and easily address safety issues before financial resources are fully expended.
RELATED READING: 5 FDA Pre-Submission Tips for Medical Device Manufacturers
4. Humanitarian Device Exemption
Humanitarian device exemption is the best FDA approval pathway for medical devices used to serve individuals affected by “orphan” diseases. Because these diseases affect less than 8,000 patients per year, it is difficult to gather clinical evidence proving the device’s therapeutic effectiveness.
As a result, applicants must demonstrate that the device performs as intended and is safe for use. Additionally, there are some unique marketing and labeling requirements that manufacturers must comply with. Finally, the FDA prevents manufacturers from profiting off of HDE products.
For a complete list of HDE requirements, visit Humanitarian Device Exemption Program: Guidance for Industry and Food and Drug Administration Staff.
How to streamline the PMA application process
What’s the best way to decrease your Class III device's time to market? It’s easy: make sure that you’ve selected and prepared for the right PMA application method from the start.
It goes without saying that investing in your medical device QMS is another simple step that you can take to streamline the PMA application process.
More and more Class III manufacturers, like TriMed, are turning to electronic quality management systems (eQMS) to accelerate and simplify their PMA submission activities.
To help you get started on the right foot, we’ve created a simple guide that outlines the top questions you need to ask prospective eQMS vendors.
Read it to learn about how to find a eQMS that aligns with your organization’s unique needs, fits within your current budget, and offers all of the features you need to scale and pass your PMA submission with flying colors.
Whatever you do, don’t leave your PMA submission to chance. Qualio offers a best-in-class eQMS for growing medical device companies, and we've helped hundreds of our customers sail through their PMA submission journeys with pre-built medical device QMS document templates.
The PMA submission is the most complex, difficult and rigorous regulatory hurdle an American medical device company can face. Careful preparation, and the right tools, are your key to success.
