Qualio vs. Cognidox: features, functionality & feedback
A balanced eQMS comparison to help you decide

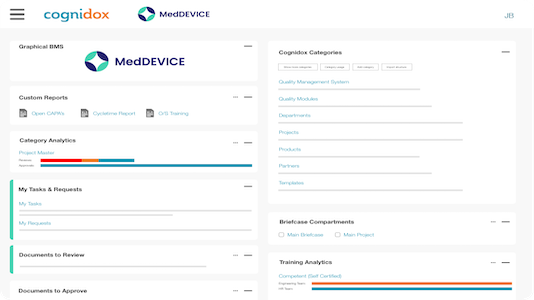
See why our customers prefer us

Features & functionality
|
|
|
|
Document management |
|
|
Training management |
|
|
CAPA, nonconformance and change control management |
|
|
Design control management |
|
|
Supplier management |
|
|
Comprehensive integration suite |
|
|
Lean and easy to use |
|
|
Designed for medical device companies |
|
|
Priced for start-ups and scale-ups |
|
|
Detailed quality analytics |
|
|
Pharmacovigilance and post-market surveillance |
|
|
Resource library of cross-referenceable data objects |
|
|
Designed for pharma and biotech companies |
|
|
Flexible workflows with form and document steps for managing any kind of quality event |
|
|
Audit-tested pre-built life science document templates |
|
|
Expert life science industry quality/regulatory support
|
|
|
AI automation: key tasks like compliance gap analysis, document change summaries and training quiz generation |
|
|
Best practice CSA methodology for rapid validation |
|
|
Native document building, editing and collaboration |
|
|
Hybrid document approach: sync with OneDrive, upload documents in original file formats, and convert to native editable formats at will |
|
|
Ranked easiest eQMS to use (G2) |
|
|
Ranked strongest customer support (G2) |
|
|
Ranked highest eQMS user satisfaction (G2) |
|
|
Configurable to scale and evolve with you |
|
Qualio: loved and trusted by 650+ companies worldwide

© Qualio — QMS for Life Sciences. All rights reserved. Read our privacy policy