InVivo Bionics is a Norwegian company bringing a hybrid software/hardware medical device to market.
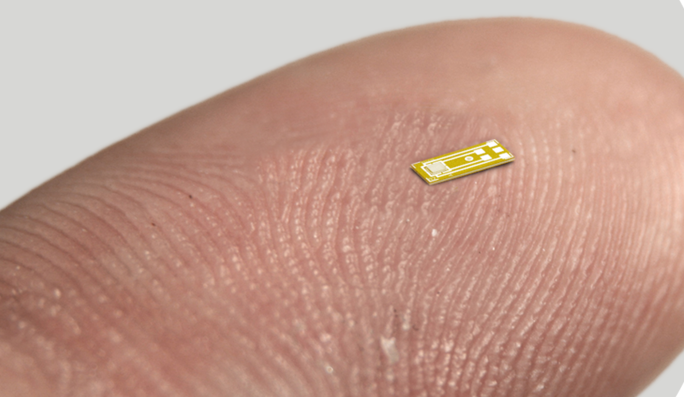
Their innovative pressure microsensor, less than 1mm across, sits within the bladder and relays real-time information via the cloud to help treat patients with urinary dysfunction.
With the EU as the primary market objective, the InVivo team is aiming for MDR and ISO 13485 accreditation, with CE marking achieved by the end of 2024.
Founder and CEO Ingelin Clausen came from SINTEF, a contract research giant based in Trondheim, to set up InVivo Bionics in 2018.
Ingelin's experience in a highly mature, successful life science organization meant she was keen to start her new project in the strongest, most quality-centric way possible.
The new InVivo Bionics team was keen to implement a system that would allow:
Audit trailing and full traceability of quality data
Centralization of the entire QMS in one tool
Control of key quality areas like design controls, CAPAs and customer feedback as the company approaches marketization
Just building a compendium of everything you need to show the notified bodies can be tricky if you're doing it manually.
We wanted to start on the right footing.
As a holistic eQMS platform housing all quality activity in an easy-to-use interface, Qualio was selected as the best eQMS for InVivo Bionics' plans.
After a white glove onboarding and validation set-up, Qualio now provides a central quality platform accessible across InVivo's operation.
The entire InVivo team, as well as external consultants and suppliers, log into Qualio and access harmonized up-to-date quality information to help guide InVivo's product to market.
Qualio provides a single quality platform enabling digital document, training and design control management.
Within Qualio Design Controls, for instance, Daniel works closely with InVivo's product engineering team and with medical subject matter experts to shape and evolve InVivo's product, from risks and requirements to verification and validation.
A centralized quality culture knits together the entire InVivo operation, with Qualio eliminating the need for paper and email clutter and making product quality the responsibility of everyone.
We didn't want to just get a tick and say, 'regulatory done'.
If we wanted to do that, we'd have purchased templates from some random company and created some documents. But that wouldn't reflect our way of operating.
We're building a quality product. So we bought a tool that gives us a quality culture. Instead of pushing quality to one department, Qualio breaks down that barrier.
Daniel plans to expand his Qualio usage as InVivo Bionics gets ever closer to market, with Qualio Events being utilised to capture and act on internal feedback for continuous improvement.
With the entire quality landscape visible within Qualio, Daniel is confident in the company's ability to bring their first product to market ahead of time - and with quality at the core.