Qualio or ETQ?
A balanced eQMS comparison to help you make the right choice
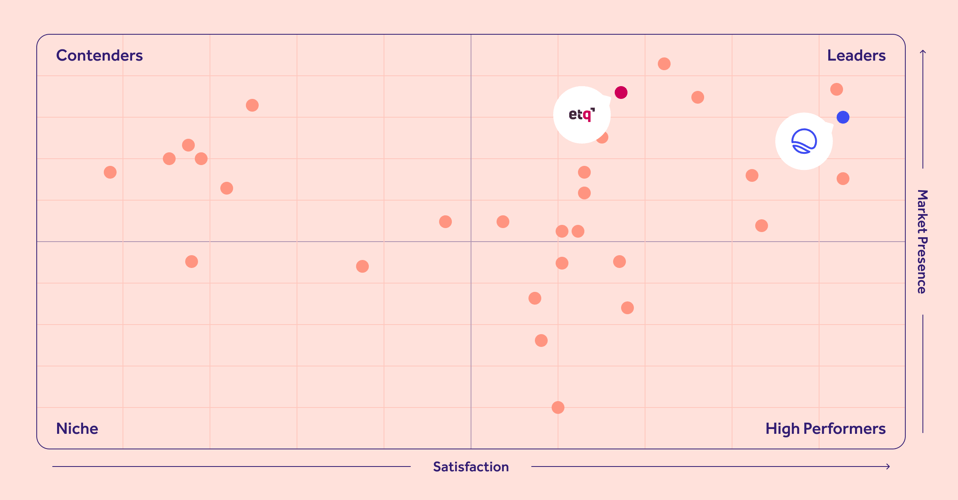
G2 Grid® Report for Quality Management (QMS) software
See why our customers prefer us
The lowdown
- Integrated, connected eQMS
- Founded 2012
-
500+ customers
-
Cloud-based
-
Supports/simplifies quality and compliance
-
Designed for SMEs
-
Life science focus
- Modular eQMS of 42 'applications': 'core' and 'sets'
-
Founded 1992
-
600+ customers
-
Cloud-based
-
Supports/simplifies quality and compliance
-
Designed and priced for enterprise businesses
-
No industry focus
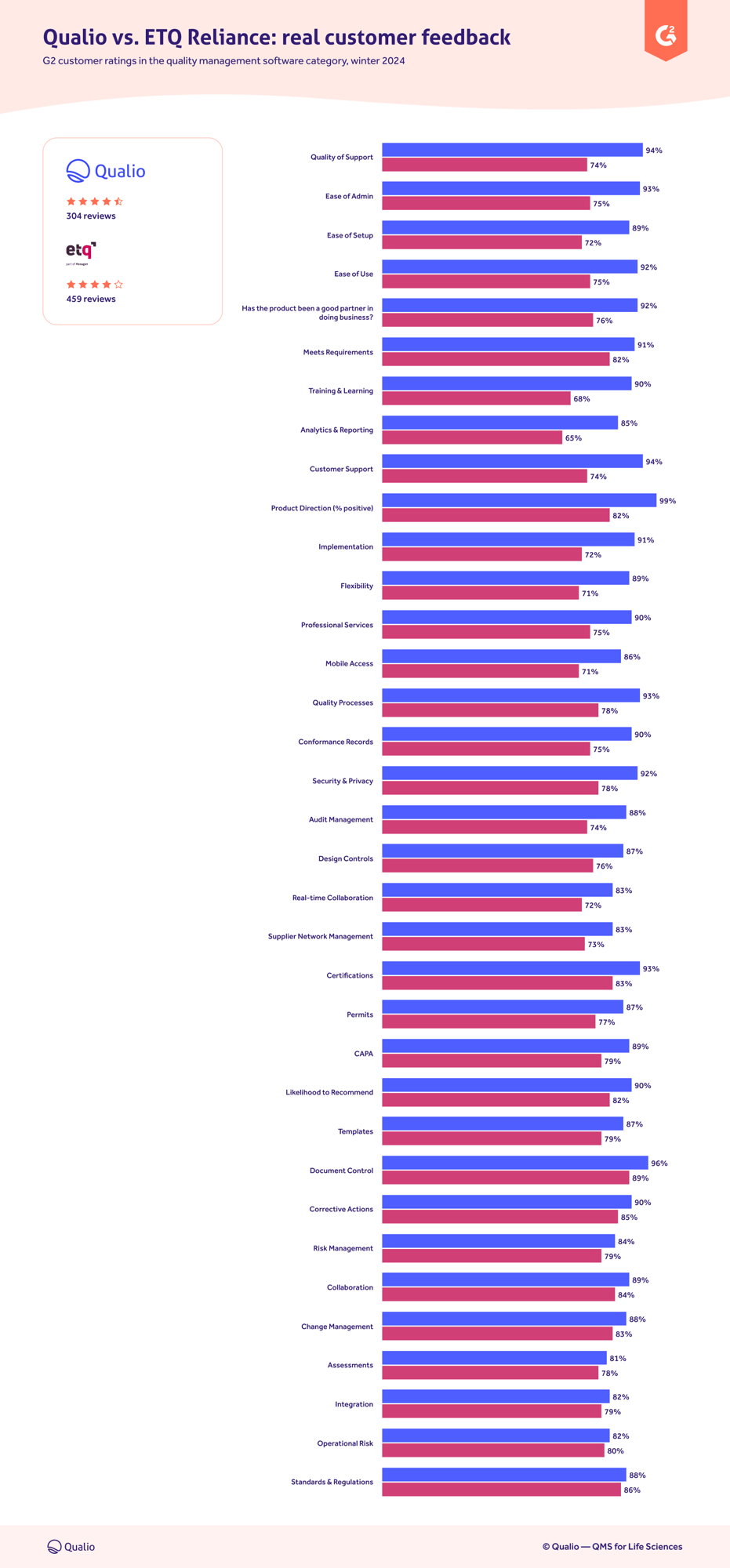
Features, functionality and usage
Document management
|
|
|
|
Training management
|
|
|
|
Quality event management
|
|
|
|
Design controls
|
|
|
|
Audit management
|
|
|
|
Equipment management
|
|
|
|
Supplier management
|
|
|
|
Ease of use
|
|
|
|
Implementation & validation
|
|
|
|
Ecosystem
|
|
|
|
Scalability
|
|
|
|
Customer focus
|
|
|
|
Choose Qualio if you:
- Are a life science company
- Want a modern, scalable, cloud-powered eQMS
- Want an integrated, life science-focused quality system with all required functionality and no hidden costs
- Want an intuitive, satisfying system backed by strong customer support
Choose ETQ if you:
- Aren't a life science company
- Are an enterprise business with high eQMS budget and no scalability needs
- Want a generic modular system with dedicated audit/equipment/meeting/permit/manufacturing management functionality
- Can commit to internal training and user support for an older, less easy-to-use platform